Draft Assessment Report On Chamaemelum Nobile (L.) All., Flos
o
EUROPEAN MEDICINES AGENCY
SCIENCE MEDICINES HEALTH
27 January 2011
EMA/HMPC/560906/2010
Committee on Herbal Medicinal Products (HMPC)
Assessment report on Chamaemelum nobile (L.) All., flos
Based on Article 16d(1), Article 16f and Article 16h of Directive 2001/83/EC as amended (traditional use)
Draft
|
Herbal substance(s) (binomial scientific name of the plant, including plant part) |
Chamaemelum nobile (L.) All., flos |
|
Herbal preparation(s) |
Liquid extract (DER 1:1), extraction solvent ethanol 70% v/v |
|
Pharmaceutical forms |
Herbal substance as herbal tea for oral use and for infusion preparation for oromucosal use. Herbal preparations in liquid dosage forms for oral use. |
Note: This draft Assessment Report is published to support the release for public consultation of the draft Community herbal monograph on Chamaemelum nobile (L.) All., flos. It should be noted that this document is a working document, not yet fully edited, and which shall be further developed after the release for consultation of the monograph. Interested parties are welcome to submit comments to the HMPC secretariat, which the Rapporteur and the MLWP will take into consideration but no 'overview of comments received during the public consultation' will be prepared in relation to the comments that will be received on this assessment report. The publication of this draft assessment report has been agreed to facilitate the understanding by Interested Parties of the assessment that has been carried out so far and led to the preparation of the draft monograph.
An agency of the European Union

7 Westferry Circus • Canary Wharf • London E14 4HB • United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7523 7051 E-mail info@ema.europa.eu Website www.ema.europa.eu
© European Medicines Agency, 2011. Reproduction is authorised provided the source is acknowledged.
Table of contents...................................................................................................................2
1.1. Description of the herbal substance(s), herbal preparation(s) or combinations thereof .3
1.2. Information about products on the market in the Member States..............................6
2.2. Information on traditional/current indications and specified substances/preparations ... 8
2.3. Specified strength/posology/route of administration/duration of use for relevant
3.1. Overview of available pharmacological data regarding the herbal substance(s), herbal
3.2. Overview of available pharmacokinetic data regarding the herbal substance(s), herbal
3.3. Overview of available toxicological data regarding the herbal substance(s)/herbal
4.1.1. Overview of pharmacodynamic data regarding the herbal substance(s)/preparation(s)
4.1.2. Overview of pharmacokinetic data regarding the herbal substance(s)/preparation(s)
1. Introduction
Chamaemelum nobile (L.) All. (syn. Anthemis nobilis L.; Anthemis odorata Lamk.; Chamaemelum odoratum Dod.; Chamomilla nobilis God.) the so-called Roman chamomile, is a perennial herb of the Asteraceae family. It is native to the Southwest Europe (France, Spain and Portugal) but the plant is present in all over Europe, North Africa and Southwest Asia. The plant is cultivated mainly in England, Belgium, France, Germany, Hungary, Poland, Bulgaria, Egypt and Argentina.
Roman chamomile reaches the height of 15 to 30 cm and generally flowers from June to September.
As a result of breeding, some of the tubular florets present in the wild plant have become ligulated, and this "double" or "semi-double" flower head forms the commercial drug. This variety (cultivar) is known from the 18th century, it is sterile and propagated vegetatively by suckering (Fauconnier et al., 1996). The white to yellowish-white flower heads are 2-3 cm in diameter and have 2-3 rows of erect, imbricate, pale green, narrowly lanceolate, membranaceous, involucral bracts. The up to 7 mm long, female ray-florets have four more or less parallel nerves, an irregular three-toothed tip, and a short, yellowish-brown ovary (achene). In the centre of the flower-head, there are a few disk-florets, but these may also be entirely absent. The base of the conical receptacle is covered with numerous oblong scales (paleae) (Bisset, 1994).
Although the Commission E did not approve Roman chamomile flower (Blumenthal et al., 1998) for an evidence-based phytotherapeutic application, the drug is listed and described in several pharmacopoeias (Barnes et al., 2002) and stated to possess carminative, anti-emetic, antispasmodic, and sedative properties. It has been used for dyspepsia, nausea and vomiting, anorexia, vomiting of pregnancy, dysmenorrhoea, and specifically for flatulent dyspepsia associated with mental stress (Bisset, 1994) (Bradley, 1992). Roman chamomile is listed by the Council of Europe as a natural source of food flavouring (category N2). This category indicates that Roman chamomile can be added to foodstuffs in small quantities, with a possible limitation of an active principle (as yet unspecified) in the final product (Barnes et al., 2002). Chamomile is commonly used as an ingredient of herbal teas. Previously, Roman chamomile has been listed as GRAS (Generally Recognised As Safe) (Leung, 1980) by the FDA. Most GRAS substances have no quantitative restrictions as to use, although their use must conform to good manufacturing practices. In case of roman chamomile, no restriction is noted (FDA, 2010).
1.1. Description of the herbal substance(s), herbal preparation(s) or combinations thereof
• Herbal substance(s)
Dried flowers of the cultivated double flowered variety of Chamaemelum nobile (L.) All. (syn. Anthemis nobilis L.) [Fam. Asteraceae]. It contains not less than 7 ml/kg of essential oil. (Ph. Eur. 6, 2008)
Dried flower heads of the cultivated double variety of Chamaemelum nobile (L.) Allioni (Anthemis nobilis L.), Compositae (Bradley, 1992).
• Herbal preparation(s)
- Liquid extract (DER 1:1, 70% ethanol) (Bradley, 1992)
- Tincture (DER 1:5, 45% ethanol) (Bradley, 1992)
• Combinations of herbal substance(s) and/or herbal preparation(s) including a description of vitamin(s) and/or mineral(s) as ingredients of traditional combination herbal medicinal products assessed, where applicable.
Not applicable.
• Constituents
Constituents derived from the flower Volatile oil
Roman chamomile flowers contain 0.6-2.4% volatile oil (Zwaving, 1982). The composition of the oil is complex, up till now more than 140 components have been identified (Fauconnier et al., 1996). In the oil the proportion of low molecular weight esters is high, which are synthesized by the esterification of a series of aliphatic C3-C6 alcohols such as n-buthanol, iso-buthanol, iso-amylacohol, and 3-methyl-pentan-1-ol with angelic acid, tiglic acid, metacryl-, n- and isobutyric, propionic and acetic acid (Zwaving, 1982) (Nano et al., 1976) (Klimes and Lamparsky, 1984). The main constituents of the volatile oil are 36.0-25.85% iso-butyl angelate, 23.7-10.9% iso-amyl iso-butyrate, 20.3-13.0% 2-methylbutyl angelate, 19.9-11.7% iso-amyl tigliate, 12% propyl tigliate, 5.3-17.9% iso-amyl angelate and 3.7-5.3% iso-butyl iso-butyrate (Chialva et al., 1982) (Antonelli and Fabbri, 1998) (Balbaa et al., 1975) (Omidbaigi et al., 2003) (Omidbaigi et al., 2004) (Inouye et al., 2006) (Tognolini et al., 2006). Furthermore, the oil contains 4% monoterpene such as a- and p-pinen, p-myrcene, limonene, y-terpinene, p-cymene, camphene, (-)-pinocarvone and (-)-trans-pinocarveol; and 1.54% sesquiterepene derivatives including p-selinene, humulene, a- and p-cubene, a- and p-caryophyllene, chamazulene, farnesene, cadinen, bisabolane and bisabolene (Fauconnier et al., 1996) (Zwaving,
1982) (Laurelle, 1959) (Chialva et al., 1982) (Duarte et al., 2005) (Inouye et al., 2006) (Tognolini et al., 2006). According to Duarte et al., (2005) the essential oil contains 4.18% bisabolene.
Sesquiterpenes (bitters)
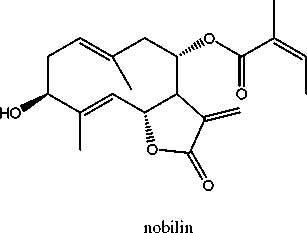
0.6% of sesquiterpene lactones of the germacranolide type (Wichtl, 2004). Sesquiterpene lactones of germacranolide type including nobilin, 3-epinobilin, 1.10-epoxynobilin, 3-dehydronobilin and hydroxyisonobilin have been identified (Holub and Samek, 1977) (Benesova et al., 1964) (Grabarczyk et al., 1977) (Samek et al., 1977) (Zwaving, 1982) (Benesova et al., 1970) (Barbetti and Casinovi, 1981).
Anthecotulide, a sesquiterpene lactone with an exocyclic methylene group having high sensitizing potential was described in A. cotula (Franke and Scilcher, 2005), but there is no report in the literature regarding to its presence in A. nobilis.
Nobilin and its derivatives are the potential contact allergens of the plant; however this is not confirmed experimentally. In one case report the epicutaneous test was negative for sesquiterpene lactones, however positive for bisabolol. The sensitization capacity of Roman chamomile is moderate (Hausen and Vieluf, 1997).
Hydroperoxides
1p-Hydroperoxyisonobilin, allylhydroperoxides have been identified from the ethanolic extract of the drug (Rucker et al., 1989) (Mayer and Rucker, 1987).
Flavonoids
Roman chamomile flowers contain 0.5% flavonoids, mainly in glycosidic form. Anthemoside (apigenin-2,3-dihydorycinnamoyl acid 7-O-p-D-glucose), cosmosiosid (apigenin 7-O-p-D-glucose), apiin (apigenin 7-O-p-D-apiosylglucoside) and chamaemeloside [apigenin 7-O-p-D-glucose-6"-(3" '-hydroxy-3"'-methyl-glutarate)], luteolin 7-O-p-D-glucose, quercetin 3-O-a-L-rhamnoside and kaempferol. The free aglycons were detected only in damaged flowers after drying (Herisset et al., 1970) (Herisset et al., 1973) (Klimes and Lamparsky, 1984) (Chaumont, 1969) (Zwaving, 1982), (Herisset et al., 1971) (Abou-Zied and Rizk, 1973) (Pietta et al., 1991) (Tschan et al., 1996).
Catechins
Catechins are responsible for the browning of the flowers during drying (Herisset et al., 1970) (Zwaving, 1982).
Coumarins
Scopolin (7-p-D-glucopyranosyl-scopoletin), umbelliferone, herniarin and, in the well dried drug, scopoletin has also been identified (Chaumont, 1969) (Zwaving, 1982) (Abou-Zied and Rizk, 1973) (Leung, 1980).
Polyacetylenes
Cis- and frans-spiroether derivatives have been detected in the flowers of Roman chamomile (Ma et al., 2007).
Phenolic acids
In the drug the glucose esters caffeic acid, ferrulic acid and anthenobilic acid were identified. In the fresh and carefully dried flowers, only the frans-caffeic acid-glucose ester was detected, whereas in the damaged flowers the frans- and cis- forms of the caffeic acid are accumulated (Chaumont, 1969) (Herisset et al., 1974).
Triterpenes and steroids
Roman chamomile flowers contain anthesterols, p-amyrin, taraxasterol, pseudotaraxasterol, p-sitosterol (Zyczynska-Baloniak et al., 1971) (Fauconnier et al., 1996).
Polysaccharides
From the aqueous extract of Roman chamomile flowers and herb acidic polysaccharides were isolated. The polysaccharide content of the dried flower is 3.9%, whereas 1.0% of the dried herb (Lukacs,
1990).
The main constituents of Roman chamomile flower are depicted in Figure 1.
Constituents derived from other plant parts
From the ethanolic extract of the leaves of Roman chamomile a sesquiterpene lactone, hydroxyisonobiline (Grabarczyk et al., 1977) (Samek et al., 1977), whereas from an apolar extract of the root (ether-petrolether 1:2, v/v) polyacetylenes, including cis- and frans- dehydromatricariaester and tiophenesetrs have been identified (Bohlmann et al., 1962) (Bohlmann and Zdero, 1966) (Bohlmann et al., 1973).
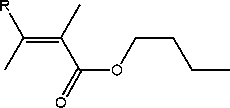
R
H n-buthyl angelate CH3 n-buthyl tigliate
Coumarins
Flavonoids
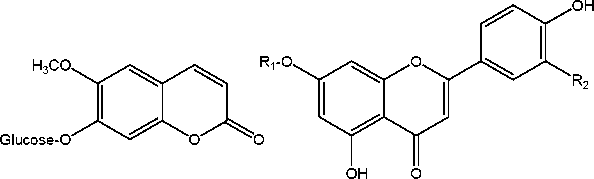
scopoletin-7-ß-D-glucose
(scopolin)
Ri
Glucose + Apiose Glucose
R2
H apiin
OH luteolin-7-ß-D-glucose
Figure 1. Main constituents of Roman chamomile flower
1.2. Information about products on the market in the Member States
Austria
The herbal substance only in multicomponent herbal teas.
Germany
The herbal substance is only available in combination products.
Regulatory status overview
|
Member State |
Regulatory Status |
Comments (not mandatory field) | |||
|
Austria |
MA |
TRAD |
Other TRAD |
Other Specify: |
Only in combination |
|
Belgium |
MA |
TRAD |
Other TRAD |
Other Specify: |
No registered or authorized products |
|
Bulgaria |
MA |
TRAD |
Other TRAD |
Other Specify: | |
|
Cyprus |
MA |
TRAD |
Other TRAD |
Other Specify: | |
|
Czech Republic |
MA |
TRAD |
Other TRAD |
Other Specify: |
No registered or authorized products |
|
Denmark |
MA |
TRAD |
Other TRAD |
Other Specify: |
No registered or authorized products |
|
Estonia |
MA |
TRAD |
Other TRAD |
Other Specify: |
No registered or authorized products |
|
Finland |
MA |
TRAD |
Other TRAD |
Other Specify: |
No registered or authorized products |
|
Member State |
Regulatory Status |
Comments (not mandatory field) | ||||
|
France |
MA |
TRAD |
Other TRAD |
Other Specify: |
No registered or authorized products | |
|
Germany |
MA |
TRAD |
Other TRAD |
Other Specify: |
Only in combination | |
|
Greece |
MA |
TRAD |
Other TRAD |
Other Specify: |
No registered or authorized products | |
|
Hungary |
MA |
TRAD |
Other TRAD |
Other Specify | ||
|
Iceland |
MA |
TRAD |
Other TRAD |
Other Specify | ||
|
Ireland |
MA |
TRAD |
Other TRAD |
Other Specify |
No registered or authorized products | |
|
Italy |
MA |
TRAD |
Other TRAD |
Other Specify: |
No registered or authorized products | |
|
Latvia |
MA |
TRAD |
Other TRAD |
Other Specify | ||
|
Liechtenstein |
MA |
TRAD |
Other TRAD |
Other Specify | ||
|
Lithuania |
MA |
TRAD |
Other TRAD |
Other Specify | ||
|
Luxemburg |
MA |
TRAD |
Other TRAD |
Other Specify | ||
|
Malta |
MA |
TRAD |
Other TRAD |
Other Specify |
No registered or authorized products | |
|
The Netherlands |
MA |
TRAD |
Other TRAD |
Other Specify: | ||
|
Norway |
MA |
TRAD |
Other TRAD |
Other Specify: |
No registered or authorized products | |
|
Poland |
MA |
TRAD |
Other TRAD |
Other Specify: |
No registered or authorized products | |
|
Portugal |
MA |
TRAD |
Other TRAD |
Other Specify | ||
|
Romania |
MA |
TRAD |
Other TRAD |
Other Specify | ||
|
Slovak Republic |
MA |
TRAD |
Other TRAD |
Other Specify |
No registered or authorized products | |
|
Slovenia |
MA |
TRAD |
Other TRAD |
Other Specify | ||
|
Spain |
MA |
TRAD |
Other TRAD |
Other Specify | ||
|
Sweden |
MA |
TRAD |
Other TRAD |
Other Specify |
No registered or authorized products | |
|
United Kingdom |
MA |
TRAD |
Other TRAD |
Other Specify: | ||
MA: Marketing Authorisation TRAD: Traditional Use Registration
Other TRAD: Other national Traditional systems of registration Other: If known, it should be specified or otherwise add 'Not Known'
This regulatory overview is not legally binding and does not necessarily reflect the legal status of the products in the MSs concerned.
1.3. Search and assessment methodology
Databases Science Direct, SciFinder, Pubmed and Web of Science were searched using the terms [Chamaemelum nobile], [Roman chamomile] and [Anthemis nobilis]. Handbooks and textbooks on the topic were also used.
Data concerning Matricaria recutita, German or Hungarian chamomile were excluded.
2. Historical data on medicinal use
2.1. Information on period of medicinal use in the Community
The name Chamaemelum was first used by Dioscurides (Hiller and Melzig, 1999). However, according to Evans (1989), it has proved impossible to trace back the drug in classical writings, because of the large number of similar Asteraceae plants.
Roman chamomile is known as a medicinal plant from the middle ages. The name Roman chamomile was first bestowed upon the plant by Joachim Camerarius in 1598, after observing it growing abundantly near Rome (Abramson et al., 2010). The European cultivation of the plant started in England in 16th century (Hiller and Melzig, 1999). The plant obtained the name "nobile" (Latin, noble) because of its therapeutic properties, which were stated to be better then those of the German chamomile (Hiller and Melzig, 1999). The double variety of the flower, which serves now as the main commercial drug, was certainly known from the 18th century (Evans, 1989). The plant was listed first in the pharmacopoeia of Wurtenberg (1741) as a carminative, painkiller, diuretic and digestive aid (Lukacs, 1990).
Augustin et al. (1948) mention Chamomillae romanae flos as a herbal drug applied both internally (dyspeptic complaints, symptoms associated with menstruation) and externally (skin problems). In the book of Rapoti and Romvary (1974), the application of the herbal drug to relieve dyspeptic complaints and flatulence is cited.
In the present Roman chamomile flower is an official drug of several pharmacopoeias including Ph. Eur. 6 (2008).
2.2. Information on traditional/current indications and specified substances/preparations
Roman chamomile-based preparations are used orally for the symptomatic treatment of gastrointestinal disorders such as epigastric bloating, impaired digestion, eructation, flatulence, and as an adjunct in the treatment of the painful component of functional digestive symptoms. Topically, it is an emollient and itch-relieving adjunct in the treatment of skin disorders and a trophic protective agent from cracks, abrasions, frostbites, chaps and insect bites. It may be used for eye irritation or discomfort of various etiologies. Furthermore, uses as an analgesic in diseases of the oral cavity, oropharynx or both and as a mouthwash for oral hygiene has been documented (Bisset, 1994) (Bruneton, 1999). The uses of Roman chamomile that are described in the Commission E monograph (Blumenthal et al., 1998) are similar: dyspepsia and inflammation of the mouth.
The drug and its extracts are ingredients of colour-lightening shampoos (perhaps due to its peroxide content) (Bruneton, 1999).
Chamomillae romanae flos is included in the British Herbal Compendium (BHC) Volume 1 published in 1992 with specified indications and posology. According to BHC, Chamomillae romanae flos has been applied in Belgium, France and Germany with specified indications at least since 1991, 1990 and 1986, respectively (Bradley, 1992).
2.3. Specified strength/posology/route of administration/duration of use for relevant preparations and indications
In the British Herbal Compendium (BHC) Volume 1, published in 1992 the indications and posologies of Roman chamomile flower are as follow:
- Internally: dyspepsia, nausea, vomiting of pregnancy irritable bowel. Posology: dried flower heads, 1.5-3 g or in infusion, three times daily; 1.5-3 ml liquid extract (DER 1:1, 70% ethanol); 3-5 ml tincture (DER 1:5 45% ethanol)
- Topically: inflammations of the skin and oral mucosa, minor wounds and abrasions. Posology: as infusion in poultices or mouthwashes; semi-solid preparations containing 5-15% of the drug or equivalent (Bradley, 1992).
According to Newall et al. (1996), the British Herbal Pharmacopoeia published in 1983 contained the dried flowerheads of Chamaemelum nobile and a liquid extract of the herbal substance (DER 1:1, 70% ethanol). The posology of the herbal substance is 1-4 g by infusion three times daily and 1-4 ml three times daily, respectively.
In the 1974 edition of the British Herbal Pharmacopoeia, dried flowerheads of Chamaemelum nobile, liquid extract (DER 1:1, 70% ethanol) are included with the indications: flatulent dyspepsia associated with mental stress. Dosage: thrice daily 1-4 g herbal substance by infusion or 1-4 ml of liquid extract thrice daily (BHP, 1974).
In Germany, according to the Standardzulassung No. 1069.99.99 (published 12. 3. 86 for a standard medicinal tea) the labelling must include: Indications: Complains such as bloatedness, flatulence and mild, spasmodic gastro-intestinal disorders; inflammations of the mouth and throat. Dosage instructions and mode of use: Pour hot water (ca. 150 ml) over a tablespoonful (2 to 3 g) of Roman Chamomile Flower and after about 10 minutes pass through a tea strainer. Unless otherwise prescribed, drink a cup of freshly prepared, warm tea 3 to 4 times daily between meals or use as a mouth and throat wash (Bradley, 1992).
In Belgium (according to Circulaire No. 367 of July 1991) the indications for this drug must be stated as:
Traditionally used in the symptomatic treatment of digestive disorders, although its activity has not been proved in accordance with the current evaluation criteria for medicines.
Or:
Traditionally used topically as an emollient and/or antalgesic and/or antiseptic, although its activity has not been proved in accordance with the current evaluation criteria for medicines.
Or:
Traditionally used topically as a soothing and antipruriginous application for dermatological affections, although its activity has not been proved in accordance with the current evaluation criteria for medicines (Bradley, 1992).
In France, according to the Bulletin Officiel No. 90/22 bis. the applications of Roman chamomile flower are as follow:
- Internally: Traditionally used in the treatment of digestive disorders such as: epigastric distension; sluggishness of the digestion; belching; flatulence. Traditionally used as adjuvant treatment for the painful component of spasmodic colitis.
- Topically: Traditionally used topically as a soothing and antipruriginous application for dermatological ailments, as protective treatment for cracks, grazes, chaps and against insect bites. Traditionally used in cases of ocular irritation or discomfort due to various causes (smoky atmosphere, sustained visual effort, bathes in the sea or swimming pool etc.). Traditionally used topically (mouth and throat washes, pastilles) as an anodyne for affections of the buccal cavity and/or the oropharynx. Traditionally used topically in mouth washes, for oral hygiene (Bradley, 1992).
Dosages for oral administration (adults) for traditional uses recommended in standard herbal reference texts are given below.
Dried flowerheads 1-4 g as an infusion, three to four times daily (Barnes et al., 2002) (Bisset,
1994).
Preparation: To prepare a decoction, add 1-4 g drug to 100-150 ml water. An infusion is prepared using 7 to 8 capitula per cup (PDR, 2000).
A 3% infusion is made for external use (Bisset, 1994).
When used as a bath additive, add 50 g to 10 litres of water. Liquid rubs are applied as poultices or washes 2 to 3 times daily (PDR, 2000).
Liquid extract 1-4 ml (1:1 in 70% alcohol), three times daily (Barnes et al., 2002).
3. Non-Clinical Data
3.1. Overview of available pharmacological data regarding the herbal substance(s), herbal preparation(s) and relevant constituents thereof
Pharmacodynamics
Anti-inflammatory effect
In the view of the similar chemical composition German and Roman chamomile are thought to possess similar pharmacological activities (Barnes et al., 2002). Few studies have been documented specifically for Roman chamomile, but the azulene compounds are reported to possess anti-inflammatory properties; their mechanism of action is thought to involve inhibition of histamine release (Barnes et al., 2002) (Abramson et al., 2010).
The anti-inflammatory effect of the polysaccharides isolated from the aqueous extract of Roman chamomile flowers and herb was tested with paw oedema test on Sprague-Dowley rats, where inflammation was generated with subplantar injection of viscarine. The flower and herb polysaccharide was administered i.p. in 10 mg/kg dose. Compared to an untreated control the polysaccharides reduced the inflammation of the paw with 36.2 and 37.7%, respectively. In the same experiment orally administered indomethacin showed 48.6% inhibition (Lukacs, 1990).
Anti-edema effect
Anti-edema effect of Roman chamomile essential oil was examined with carrageenan induced paw edema test in male Wistar rats. Intraperitoneal (i.p.) injection of 350 mg/kg volatile oil inhibited the edema formation of the paw with 22.8-38.7% after two hours and with 38.0-43.0% after 3 hours. Indomethacin (5 mg/kg, i.p.) under the same conditions showed 73.7% and 66.7% inhibition, respectively (Rossi et al., 1988) (Melegari et al., 1988). Due to the high dosage the results can not be interpreted (Hansel et al., 1993).
Antimicrobial activity
The volatile oil showed activity (filter paper diffusion test) against Gram-positive bacteria, especially Bacillus subtilis, B. anthracis, Micrococcus glutamicus, B. sacchrolyticus, B. thuringiensis, Sarcina lutea, B. stearothermophilus, Lactobacillus plantarum, Staphylococcus aureus, Staphylococcus sp. and L. casei, whereas the oil showed no activity against Gram-negative bacteria species including Salmonella group B, Citrobacter sp., Enterobacter sp., Esheria. coli, Pseudomonas sp., Salmonella saintpaul and Salmonella weltevreden. The Roman chamomile volatile oil inhibited the growth of dermatophytons, Alternaria sp., Aspergillus fumigatus and A. parasiticus (Hansel et al., 1993). In the same study the volatile oil was inactive against Candida albicans, Cryptococcus neoformans, Histoplasma capsulatum and Aspergillus niger (Hansel et al., 1993).
According to Piccaglia et al. (1993) Roman chamomile essential oil possessed a moderate antibacterial
activity, determined with agar diffusion method, against Flavobacterium suaveolens, Clostridium sporogenes and Micrococcus luteus.
Roman chamomile essential oil was moderately effective against Gram-positive and Gram-negative bacteria using a disk diffusion method. Moreover, the oil showed activity against C. albicans and Rhizopus oligosporus. In the same study, Roman chamomile oil was the most active against T7 and SA phage viruses (Chao et al., 2000).
Moderate activity of Roman chamomile oil against C. albicans (MIC=0.8 mg/ml) was determined by microplate method, whereas the alcoholic extract of the plant was ineffective (Duarte et al., 2005). Strong activity of Roman chamomile flower essential oil was detected against strains of Gram-positive (S. aureus, Enterococcus faecalis) and Gram-negative (E. coli, Proteus vulgais, Klebsiella pneumoniae and Salmonella sp.) bacteria as well as against C. albicans using agar diffusion and agar dilution methods. The effect was attributed to both the main and minor ester constituents of the oil (Bail et al., 2009).
A blended essential oil, containing Roman chamomile flower, ylang ylang, spruce and lavender oils showed potent activity against MRSA determined with a disk diffusion assay. None of the tested oils showed significant activity when tested alone suggesting a synergistic effect of the combined oils, however a definitive proof would require further testing (Chao et al., 2008).
Hydroperoxides [1: Z-2-methyl-2-butyric acid-(2-hydroperoxy-2-methyl-3-butenyl) ester, 2: Z-2-methyl-2-butyric acid-(3-hydroperoxy-2-methylidenebutyl) ester], isolated from the ethanolic extract of the Roman chamomile flowers, showed antibacterial activity against E. coli, P. aeruginosa and E. faecalis. The MIC values of compound 1 were 256 pg/ml agains E. coli and 512 pg/ml agains P. aeruginosa. The MIC values of compound 2 were 512 and 128 pg/mL, respectively (Hansel et al., 1993).
Ethyl acetate extract of Roman chamomile leaf showed potent vapour and contact activity against Trichophyton mentagrophytes determined by a box vapour and agar diffusion assay, respectively. The composition of the extract was similar to that of the Roman chamomile volatile oil (Inouye et al., 2006).
Aqueous extract of Roman chamomile leaf completely inhibited the growth of Aspergillus candidus, A. niger, Penicillium sp. and Fusarium culmorum in a concentration of 92 g/ml media (Magro et al.,
2006).
Cytostatic activity
Nobilin, 1,10-epoxynobilin, 3-dehydronobilin and hydroxyisonobilin, isolated from Roman chamomile flower, showed in vitro cytostatic activity against human HeLa (cervix carcinoma cell line) and KB (nasopharyngeal carcinoma) cell lines (Holub and Samek, 1977). ED50 for hydroxyisonobiline were 0.5 pg/mL (1.5 x 10-6 M) and 1.23 pg/mL (3.5 x 10-6 M) for HeLa and KB cell lines, respectively (Holub and Samek, 1977), (Samek et al., 1977) (Grabarczyk et al., 1977).
Antioxidant activity
Antioxidant activity of Roman chamomile essential oil, acetone oleoresins (AO = dried acetone extract of fresh Roman chamomile flowers, containing the essential oil) and deodorized acetone extract (DAE = dried acetone extract of Roman chamomile flowers, from which the volatile oil content was primarily removed by hydrodistillation) has been investigated with various methods, including p-carotene bleaching assay, rapeseed oil stabilizing assay (peroxide value, oxygen absorption and UV absorption of formed aldehydes and ketones), measurement of free radical scavenging activity with different radicals (DPPH*, ABTS*+ and *OH), assessment of the influence on the enzyme xanthine oxidase,
reducing power measured on Fe3+ and Fe2+ chelating effect (Piccaglia et al., 1993) (Bandoniene et al., 2000) (Povilaityte and Venskutonis, 2000), (Venskutonis et al., 2005), (Podsedek et al., 2009).
Roman chamomile oil had high antioxidant activity in the p-carotene bleaching assay (Piccaglia et al., 1993) showed significant inhibitory effect against hydroxyl radicals and acted as a ferrous ion chelator (Podsedek et al., 2009).
Roman chamomile flower AO significantly inhibited the xanthine oxidase enzyme but showed no significant free radical scavenging activity in the DPPH* and ABTS*+ assays or reducing power converting Fe3+ to Fe2+ (Venskutonis et al., 2005). Moreover, Roman chamomile flower DEA and AO significantly stabilized rapeseed oil during storing, measured by all three methods in a concentration of 0.1% w/w (Bandoniene et al., 2000) (Povilaityte and Venskutonis, 2000).
Insecticidal activity
Roman chamomile volatile oil showed high activity against the whitefly (Trialeurodes vaporariorum) nymphs at 0.0047 and 0.0093 pg/ml air using an impregnated filter paper test, whereas it was ineffective against the adult or egg forms. The results indicated that the mode of delivery of these oils was largely a result of action in the vapour phase, they might be toxic through penetration via the respiratory system (Choi I et al., 2004).
Antiplatelet activity
Roman chamomile oil dose dependently inhibited in vitro the induced platelet aggregation in guinea pig plasma, although with a modest potency. The oil showed no effect on clot retraction (Tognolini et al., 2006).
Mobility decreasing effect
Effect of subcutaneously (350, 1250 and 2500 mg/kg) and i.p. (175 and 350 mg/kg) administered Roman chamomile oil was measured in male Wistar rats. The essential oil of Roman chamomile decreased the mobility of male Wistar rats with 51-76% compared to untreated control. The effect lasted for 50 minutes (Melegari et al., 1988). Due to the high dosage the results cannot be interpreted (Hansel et al., 1993).
Diuretic effect
Effect of subcutaneously (350, 1250 and 2500 mg/kg) and i.p. (175 and 350 mg/kg) administered Roman chamomile oil was measured in male Wistar rats. The oil caused a reduction in diuresis by 50% in 350 mg/kg or lower doses, whereas in higher doses an opposite effect was observed (Melegari et al., 1988). Due to the high dosage the results cannot be interpreted (Hansel et al., 1993).
Repeated oral administration of Roman chamomile flower aqueous extract (140 mg/kg, for 20 days) produced significant increase in urinary output and electrolyte (Na+, K+, Cl-) excretion from the eighth day to the end of the treatment in spontaneously hypertensive rats (Zeggwagh et al., 2009).
Hypotensive effect
Single oral administration of Roman chamomile aqueous extract (140 mg/kg) produced a slight but significant reduction in systolic blood pressure in spontaneously hypertensive rats after 24 hours of the administration. Daily oral administration of the extract in the same dose for three weeks produced a significant reduction in baseline arterial blood pressure starting from day eight without affecting the heart rate. In both, the single and repeated oral administration of Roman chamomile aqueous extract, the underlying hypotensive mechanism seems to be independent from plasmatic angiotensin convertase enzyme activity. However, the effect of single oral administration of Roman chamomile extract seemed to be independent of diuresis, whereas in case of the repeated extract administration the decrease in the blood pressure may be due to an increased water and electrolyte (sodium, potassium and chloride) excretion (Zeggwagh et al., 2009).
Hypoglycaemic effect
Chamaemeloside, an apigenin glycoside containing a hydroxymethylglutaric acid (HMG) moiety, had no effect on glucose uptake in culture L6 muscle cells, but decreased the glucose plasma levels of Swiss-Webster mice by 19.2% and 31.9% at dosages of 125 and 250 mg/kg, respectively. Chamaemeloside exerted its effect 4 hours after i.p. administration. Chamaemeloside was also administered orally to normal mice to assess its effect on interprandial glycaemia and oral glucose tolerance. Although, the interprandial glycaemia was not affected, chamaemeloside significantly improved glucose tolerance 4 hours after administration. Chamaemeloside might influence glucose homeostasis via multiple mechanisms but the results on cultured L6 cells might exclude insulin-like activity. It is possible that HMG acid is being liberated from chamaemeloside and the observed activity is modulated in a similar fashion to that proposed for HMG itself (Witherup et al., 1995) (Konig et al., 1998). It has to be noted that the concentration of chamaemeloside in the herbal drug is much too low (0.05-0.1%) to have any significant effect on plasma glucose concentrations in man in the currently recommended daily dose (6 g) (Konig et al., 1998).
In a further study the effect on blood glucose concentrations and basal insulin levels in normal and streptozotocin-induced diabetic rats (STZ) was examined. Single dose and daily oral administration for 15 days of the aqueous extract of the aerial part of Roman chamomile at a dose of 20 mg/kg body weight was administered. Single oral administration of Roman chamomile aqueous extract reduced blood glucose levels significantly 6 hours after administration in normal and STZ diabetic rats. Furthermore, blood glucose levels were decreased significantly in normal and STZ diabetic rats, respectively, after 15 days of treatment. Basal plasma insulin concentrations remain unchanged after treatment in both normal and STZ diabetic rats so the mechanism seems to be independent of insulin secretion (Eddouks et al., 2005).
3.2. Overview of available pharmacokinetic data regarding the herbal substance(s), herbal preparation(s) and relevant constituents thereof
No data available.
3.3. Overview of available toxicological data regarding the herbal substance(s)/herbal preparation(s) and constituents thereof
Roman chamomile essential oil is relatively non-toxic following an acute exposure. Acute oral LD50 in rats was greater than 5 g/kg. Dermal application of 5 g/kg to rabbits did not result in any deaths. Undiluted Roman chamomile oil applied to the backs of hairless mice produced no irritating effects. When applied full strength to intact or abraded rabbit skin for 24 hours under occlusion, Roman chamomile oil was only mildly irritating. Animal studies have indicated the oil to be either mildly or non-irritant, and to lack any phototoxic effects (Opdyke, 1974).
3.4. Overall conclusions on non-clinical data
Limited pharmacological data are available for Roman chamomile, mainly dealing with the antibacterial and antioxidant effect of the essential oil. The limited amount of toxicity data for Roman chamomile requires further investigation (Barnes et al., 2002).
4. Clinical Data
4.1. Clinical Pharmacology
4.1.1. Overview of pharmacodynamic data regarding the herbal substance(s)/preparation(s) including data on relevant constituents
No relevant data available.
4.1.2. Overview of pharmacokinetic data regarding the herbal substance(s)/preparation(s) including data on relevant constituents
No relevant data available.
4.2. Clinical Efficacy
See below.
No data available.
4.2.2. Clinical studies (case studies and clinical trials)
Curative and preventive effects of externally applied emulsions containing alcoholic extract of Roman chamomile, alcoholic extract of German chamomile or steroid were examined in a placebo controlled trial in 20 volunteers. The chamomile extracts were included in model O/W emulsions at concentration of 5%. Preventive effectiveness was ascertained by using a sun stimulator to determine the threshold erythema time of skin areas which have regularly been treated with the test emulsion, emulsion containing 0.15% triamcinolone acetonide or placebo. 15-20 minutes before the radiation the same skin part has been treated with one of the emulsions or placebo. As a control a commercial product (COLIPA Low-Standard, SPF 4.0-4.4) has been used.
Curative effectiveness was ascertained by comparing the subsidence of UV-induced erythemas on skin areas after 16-24 hours, which have been treated with test emulsion, emulsion containing 0.15% triamcinolone acetonide or placebo for two weeks. Whereas no preventive effect of the chamomile containing emulsions was found, the chamomile extract containing emulsions showed significant effectiveness to enhance the regeneration of skin erythemas compared to placebo. In particular Roman chamomile leads to a faster soothing of skin that has been irritated with by UV irradiation (Schrader et al., 1997).
There is no data in the publication about the criteria and mode of group formation, statistics used and about the fact, whether the trial was blinded or not.
A randomized, uncontrolled study was performed to assess the effects of massage and aromatherapy massage on cancer patients in a palliative care setting. One hundred and three patients were randomly allocated to receive massage using carrier oil (massage) and carrier oil plus Roman chamomile essential oil (aromatherapy massage). Outcome measurements included the Rotterdam Symptom Checklist (RSCL), the State-Trait Anxiety Inventory (STAI) and a semi-structured questionnaire, administered 2 weeks post-massage. There was a statistically significant reduction in anxiety in each massage group on the STAI. Scores improved significantly on RSCL including the psychological, quality of life, severe physical and severe psychological subscales for the aromatherapy massage group, whereas the improvement in the massage group did not reach the level of statistical significance. It was concluded that massage with or without essential oils appeared to reduce levels of anxiety. The addition of Roman chamomile essential oil seemed to enhance the effect of massage and to improve physical and psychological symptoms, as well as quality of life. The results of this study may not be easily generalized because the sample number was small, no entrance criteria was provided so the anxiety level of the patients entered to the study was varying and there was no control group (Wilkinson et al., 1999).
4.2.3. Clinical studies in special populations (e.g. elderly and children)
No relevant data available.
4.3. Overall conclusions on clinical pharmacology and efficacy
Clinical research assessing the effects of Roman chamomile is very limited, and rigorous randomised controlled clinical trials are required. The effectiveness for the traditional and claimed uses is not documented (Blumenthal et al., 1998), therefore a monograph on well-established use is not proposed. However, in view of the similar chemical compositions, many of the activities described for German chamomile (Matricaria recutita L.) are thought to be applicable to Roman chamomile and thus support the traditional uses (Barnes et al., 2002).
5. Clinical Safety/Pharmacovigilance
5.1. Overview of toxicological/safety data from clinical trials in humans
No relevant data were reported.
5.2. Patient exposure
Two clinical trials evaluated in the assessment report comprised altogether 123 patients (Schrader et al., 1997) (Wilkinson et al., 1999).
5.3. Adverse events and serious adverse events and deaths
Two cases of contact-allergic nipple eczemas in breastfeeding women were reported from England: both women had applied Kamillosan® ointment (suppliers Norgine Limited), which in the product commercialized in the UK contains extracts and oil of Roman chamomile instead of German chamomile, and both patients had a +++ reaction to chamomile oil 0.1% and negative reactions to other ingredients (McGeorge and Steele, 1991). However, it is questionable whether the product mentioned in the article contains Roman chamomile. Sensitization from compresses containing Roman chamomile has been reported in two sesquiterpene lactone mix positive patients from Belgium and Portugal, whereas a French patient sensitized from both compresses of Roman chamomile and a homoeopathic preparation containing Roman chamomile oil was negative to sesquiterpene lactone mix (Pereira et al., 1997) (Giordano-Labadie et al., 2000) (Bossuyt and Dooms-Goossens, 1994). Presumably, cases of sensitization are primarily caused by sesquiterpene lactones, but prolonged or repeated topical application seems necessary to sensitize with the tea because of its lower content of allergens (Pereira et al., 1997). Three of the above five patients reported above had weak positive reactions to fragrance allergens and two to balsam of Peru (Myroxylon pereirae), which may reflect to cross-reactions with sesquiterpenes (Bossuyt and Dooms-Goossens, 1994) (Giordano-Labadie et al., 2000) (McGeorge and Steele, 1991). However, the connection between Roman chamomile oil hypersensitivity and Peru balsam and fragrance allergen allergy is hypothetic, reflecting the opinion of Paulsen (Paulsen, 2002).
Idiosyncratic allergic reaction (head rush, tachycardia and nausea) has been reported in case of a nursing student after inhaling Roman chamomile volatile oil dropped to a strip on an aromatherapy class exercise. During the 10-year lecturer practice of the author this was the only case of allergic reaction to Roman chamomile oil (Maddocks-Jennings, 2004).
5.4. Laboratory findings
No data reported.
5.5. Safety in special populations and situations
Pregnancy
There have been no formal studies on the effects of Roman chamomile on pregnant women. In a systematic review on potential value of plant sources of antifertility agents, Farnsworth presents folkoric data from papers published in the early 1960's. One of the cited sources mentions the abortive effect of the plant (plant part not defined). Two other references report emmenagogue effect of the volatile oil or the whole plant. No further details are mentioned regarding the dosage (Farnsworth et al., 1975). However, due to its theoretical properties as an abortifacient and emmenagogue, most experts agree that excess ingestion of Roman chamomile should be avoided during pregnancy. Roman chamomile has a class 2b safety rating from the American Herbal Products Association, advising not to use the plant during pregnancy, because of its potential abortifacient effects when taken at high doses due to its action on uterine smooth muscle and tendency to induce menstruation (Abramson et al., 2010). There are no reliable data from human studies or case reports on the emmenagogue and abortive effect of Roman chamomile, so in therapeutic posology the appearance of such adverse effects is not plausible.
5.6. Overall conclusions on clinical safety
No health hazards or side effects are known in conjunction with the proper administration of designated therapeutic dosages. The drug possesses a small potential for sensitization. In view of the documented allergic reactions and cross-sensitivities, Roman chamomile should be avoided by individuals with a known hypersensitivity to any members of the Asteraceae family. In addition, Roman chamomile may precipitate an allergic reaction or exacerbate existing symptoms in susceptible individuals (e.g. asthmatics) (Paulsen, 2002).
The potential for preparations of Roman chamomile to interact with other medicines administered concurrently, particularly those with similar or opposing effects, should be considered (particularly where oral preparations of Roman chamomile are used).
Coumarin compounds detected so far in Roman chamomile do not possess the minimum structural requirements (a C-4 hydroxyl substituent and a C-3 non-polar carbon substituent) for anticoagulant activity (Barnes et al., 2002).
Roman chamomile oil has also been reported to be non-irritant to human skin (Opdyke, 1974). No photosensitising effect of the coumarin compounds isolated from Roman chamomile has been observed (Barnes et al., 2002).
Based on folkloric evidences, Roman chamomile is reputed to be an abortifacient and emmenagogue (Farnsworth et al., 1975). In view of this and the potential for allergic reactions, the excessive use of Roman chamomile during pregnancy and lactation should be avoided (Barnes et al., 2002).
6. Overall conclusions
The use of C. nobile has a long tradition in Europe. The provided clinical and non-clinical data do not fulfil the requirements of a well-established medicinal use with recognised efficacy and an acceptable level of safety of Roman chamomile products.
Roman chamomile is considered to have an impact on the protection of public health on the basis of the long medicinal tradition in the specified conditions. Therefore Roman chamomile products may be considered as traditional herbal medicinal products.
Toxicological data on Roman chamomile is very limited. Nonetheless, neither the chemical composition nor the long-term widespread use in the European Union suggest that there is a high risk associated with the use of Roman chamomile products. Yet, due to the lack of data on acute and chronic toxicity, repeated dose toxicity, genotoxicity, mutagenicity, carcinogenicity, reproductive and developmental toxicity, the safety of the therapeutic application of C. nobile flower cannot be confirmed from such testing.
The drug possesses a small potential for sensitization. In view of the documented allergic reactions and cross-sensitivities, Roman chamomile should be avoided by individuals with a known hypersensitivity to any sesquiterpene containing members of the Asteraceae family (Barnes et al., 2002). However, considering the long-standing traditional use of Roman chamomile flower in Europe and the fact that the chemical composition, indications and uses of the Roman and German chamomile are similar (Barnes et al., 2002) (Hansel et al., 1993) (Bisset, 1994), the benefit/risk balance of the medicinal application of good quality products is positive.
Annex
List of references
Page 17/17
Assessment report on Chamaemelum nobile (L.) All., flos
EMA/HMPC/560906/2010