Draft Assessment Report On Myroxylon Balsamum (L.) Harms Var. Pereirae (Royle) Harms, Balsamum
o
EUROPEAN MEDICINES AGENCY
SCIENCE MEDICINES HEALTH
24 November 2015
EMA/HMPC/712648/2014
Committee on Herbal Medicinal Products (HMPC)
Assessment report on Myroxylon balsamum (L.) Harms var. pereirae (Royle) Harms, balsamum
Draft
Based on Article 10a of Directive 2001/83/EC as amended (well-established use)
Based on Article 16d(1), Article 16f and Article 16h of Directive 2001/83/EC as amended (traditional use)
|
Herbal substance (binomial scientific name of the |
Myroxylon balsamum (L.) Hams var. pereirae |
|
plant, including plant part) |
(Royle) Harms, balsamum |
|
Herbal preparation(s) |
Not applicable |
|
Pharmaceutical form(s) |
Not applicable |
|
Rapporteur(s) |
P. Claeson |
|
Assessor(s) |
E. Svedlund |
|
Peer-reviewer |
W. Knoss |
Note: This draft assessment report is published to support the public consultation of the draft public statement on Myroxylon balsamum (L.) Hams var. pereirae (Royle) Harms, balsamum. It is a working document, not yet edited, and shall be further developed after the release for consultation of the public statement. Interested parties are welcome to submit comments to the HMPC secretariat, which will be taken into consideration but no 'overview of comments received during the public consultation' will be prepared on comments that will be received on this assessment report. The publication of this draft assessment report has been agreed to facilitate the understanding by Interested Parties of the assessment that has been carried out so far and led to the preparation of the public statement.
An agency of the European Union

30 Churchill Place • Canary Wharf • London E14 5EU • United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact
© European Medicines Agency, 2015. Reproduction is authorised provided the source is acknowledged.
Table of contents...................................................................................................................2
1.1. Description of the herbal substance(s), herbal preparation(s) or combinations thereof ..4
2.1.1. Information about products on the market in the EU/EEA Member States.................5
2.1.2. Information on products on the market outside the EU/EEA....................................5
2.2. Information on documented medicinal use and historical data from literature..............5
3.1. Overview of available pharmacological data regarding the herbal substance(s), herbal
3.2. Overview of available pharmacokinetic data regarding the herbal substance(s), herbal
3.3. Overview of available toxicological data regarding the herbal substance(s)/herbal
4.1.1. Overview of pharmacodynamic data regarding the herbal substance(s)/preparation(s)
4.1.2. Overview of pharmacokinetic data regarding the herbal substance(s)/preparation(s)
Assessment report on Myroxylon balsamum (L.) Harms var. pereirae (Royle) Harms, balsamum
5.5.7. Effects on ability to drive or operate machinery or impairment of mental ability......16
1. Introduction
1.1. Description of the herbal substance(s), herbal preparation(s) or combinations thereof
• Herbal substance
Peru balsam is obtained from the scorched and wounded trunk of Myroxyion balsamum (L.) Harms var. pereirae (Royle) Harms. It contains not less than 45.0-70.0 % m/m of esters, mainly benzyl benzoate and benzyl cinnamate (European Pharmacopeia 01/2008:0754).
There is also a similar herbal substance, Tolu balsam (Balsamum tolutanum), which should not be mixed-up with Peru balsam. Tolu balsam is the oleo-resin obtained from the trunk of the variety Myroxyion balsamum (L.) Harms var. balsamum. In the European Pharmacopeia monograph, Tolu balsam contains 25-50 % m/m of free or combined acids, expressed as cinnamic acid (dried drug) (European Pharmacopeia 01/2008:1596).
• Herbal preparation(s)
Not applicable.
Constituents
The main constituents of Peru balsam (55-66 %) are esters of cinnamic and benzoic acids, especially benzyl cinnamate (cinnamein), cinnamyl cinnamate (styracine) and benzyl benzoate. Small amounts of vanillin and free cinnamic acid are also present (Samuelson and Bohlin, 2009)
Peru balsam contains 50-64 % of a high-boiling volatile oil called cinnamein, along with 20-28 % of resin. The volatile oil consists mainly of benzoic and cinnamic acid esters such as benzyl benzoate, benzyl cinnamate and cinnamyl cinnamate (styracine), with small amounts of nerolidol, free benzyl alcohol, and free benzoic and cinnamic acids also present. In addition, traces of styrene, vanillin, and coumarin have been identified in the material (Leung, 1996).
• Combinations of herbal substance(s) and/or herbal preparation(s) including a description of vitamin(s) and/or mineral(s) as ingredients of traditional combination herbal medicinal products assessed, where applicable.
1.2. Search and assessment methodology
Medicinal use of Peru balsam has been documented in several handbooks that are included in the list of references. PubMed, EMBASE and TOXNET were searched in October 2014 using the terms [Peru balsam], [Balsam of Peru], [Peruvian balsam], [Myroxylon pereirae], and [Myroxylon balsamum], separately. The abstracts of the references retrieved were screened manually and all articles considered relevant were assessed and included in the list of references. TOXNET was also searched using the terms [benzyl benzoate], [benzyl cinnamate], [cinnamyl cinnamate], [vanillin], [nerolidol], [styrene], [farnesol] and [peruviol].
The EudraVigilance database and Vigisearch database of the World Health Organization were searched in October 2014 using the term [Peru balsam], [Balsamum peruvianum], [Myroxylon pereirae], and [Myroxylon balsamum].
Data was also provided by the EMA on behalf of interested parties.
Additionally, the European Commission's database on cosmetic ingredients (Cosing) was searched in October 2014 for information on [Myroxylon].
2. Data on medicinal use
2.1. Information about products on the market
2.1.1. Information about products on the market in the EU/EEA Member States
Information on medicinal products marketed in the EU/EEA
Table 1: Overview of data obtained from marketed medicinal products
|
Active substance |
Indication |
Pharmaceutical form |
Regulatory Status |
|
Myroxylon balsamum (L.) Harms var. pereirae (Royle) Harms, balsamum |
Traditional herbal medicinal product used for local use as additional emollient treatment and antipruritic for skin disorders, as trophic barrier for the treatment of cracks, grazes and chapping. |
Ointment, 4.95 g/100 g Cutaneous use. For adults and adolescents over 12 years of age. Apply two times daily. Duration of use: 1 week |
Since 1944, France, TU |
This overview is not exhaustive. It is provided for information only and reflects the situation at the time when it was established.
Information on relevant combination medicinal products marketed in the EU/EEA
Not applicable.
Information on other products marketed in the EU/EEA (where relevant)
2.1.2. Information on products on the market outside the EU/EEA
2.2. Information on documented medicinal use and historical data from literature
Peru balsam, included in the U.S.P. from 1820, is obtained from the tree Myroxylon pereirae. The tree is abundant in San Salvador, but the name "Peru balsam" refers to the early import of the balsam into Spain via the port of Lima in Peru. In the 17th century the drug appeared in German pharmacies. Applied externally, Peru balsam has been described as an antiseptic and vulnerary, either alone, in alcoholic solution, or in the form of an ointment. Internally it has been used as a stimulating expectorant, but the internal use is rather rare (Claus, 1956).
Peru balsam has been described in many national pharmacopoeias, for example in the national pharmacopoeias of Argentina, Austria, Belgium, Brazil, Chile, France, Germany, Italy, Japan, Mexico, Netherlands and Spain. It has been reported to have a very mild antiseptic action. Diluted with equal part of castor oil, it has been used as an application to bedsores and chronic ulcers. As an ointment (12.5 % in simple ointment) it has been used in the treatment of eczema and pruritus. It has been used as an ingredient of some rectal suppositories for the symptomatic relief of haemorrhoids. Peru balsam was formerly used as an ointment in the treatment of scabies, but it has been superseded for this purpose by benzyl benzoate (Martindale, 1972).
Peru balsam has been used as an antiseptic dressing for wounds and as a parasiticide (Trease and Evans, 1978).
Peru balsam has been used as a local protectant, rubefacient, parasiticide in certain skin diseases, antiseptic, and vulnerary, and applied externally as an ointment, or in alcoholic solutions; internally, rarely used as an expectorant (Duke, 1985).
Peru balsam has been locally used on infected and poorly healing wounds, burns, decubitus, frostbites, prostheses pressure points, haemorrhoids. Dose: 5-20 %, but not higher than 10 % for extended surface treatment (Hansel, 1993).
Peru balsam has been used in topical preparations for the treatment of wounds, indolent ulcers, scabies, diaper rash, haemorrhoids, anal pruritus, bedsores, and intertrigo (Leung, 1996).
Peru balsam has been used as a vulnerary and has been reported to be antiseptic. It is irritating when taken orally, and has therefore only been used externally. It has been used for the treatment of burns, frostbites, chaps, cracks, erythema, pruritus, ulcers, infected dermatitis, and other minor wounds. It has also been used in suppositories for the symptomatic treatment of pain, pruritus, and feelings of congestion associated with the acute attack of piles and other anal disorders. It has been an ingredient of preparations designed to be inhaled after dispersion in hot water (Bruneton, 1999).
Table 2: Overview of historical data
|
Herbal preparation |
Documented use / Traditional use |
Pharmaceutical form |
Reference |
|
Myroxylon balsamum (L.) Harms var. pereirae (Royle) Harms, balsamum |
For infected and poorly healing wounds, for burns, decubitus ulcers, frost bite, ulcus cruris, bruises caused by prostheses, haemorrhoids. |
Cutaneous use Preparations containing 5-20 %, for extensive surface application not more than 10 % |
Blumenthal et al., 1990; Hänsel, 1993 |
|
Vulnerary |
Applied externally, either alone, in alcoholic solution, or in the form of an ointment |
Claus, 1956; Duke, 1985 | |
|
Wound balm |
Usual dose: 0.5-2 g daily |
Madaus, 1938 | |
|
Eczema and pruritus |
Cutaneous use Ointment containing 12.5 % |
Martindale, 1972 and 1993 | |
|
Wound healing |
Cutaneous use Diluted 1:2 in oil (e.g. ricin oil) or in 5-10 % ointment |
Ljungdahl, 1953 | |
|
Scabies |
Cutaneous use Undiluted or diluted 5-10:100 vaseline or 10-15:200 spir. dilut. |
Ljungdahl, 1953 |
2.3. Overall conclusions on medicinal use
The traditional medicinal use of Peru balsam has been documented in several medicinal handbooks throughout a period of at least 30 years, including at least 15 years within the EU.
The strength and posology are summarised in table 3.
Table 3: Overview of evidence on period of medicinal use
|
Herbal preparation Pharmaceutical form |
Indication |
Posology, Strength |
Period of medicinal use |
|
Myroxylon balsamum (L.) Harms var. pereirae (Royle) Harms, balsamum |
Traditional herbal medicinal product for the symptomatic relief of minor inflammations of the skin and as an aid in healing of minor wounds. The product is a traditional herbal medicinal product for use in the specified indication exclusively based upon long-standing use. |
Cutaneous use Adults and elderly Daily dose 5-10 % balm in semisolid preparations. To be applied once daily. Duration of use: 1 week |
Since 1944 in France, Ljungdahl, 1953, Claus, 1956, Martindale, 1972, Blumenthal et al, 1990, Hänsel, 1993 |
3. Non-Clinical Data
3.1. Overview of available pharmacological data regarding the herbal substance(s), herbal preparation(s) and relevant constituents thereof
3.1.1. Primary pharmacodynamics
In agar diffusion assays, an antibacterial effect has been demonstrated in gram-positive as well as negative strains. Also a fungicidal or fungistatic potential have been demonstrated (Hansel, 1993). The concentrations used were not reported.
Peru balsam has been reported to eradicate biofilms of Pseudomonas spp. (MIC 2.5 %) and Staphylococcus aureus (MIC 2.5 %) (Kavanaugh and Ribbeck, 2012).
In radiogenic skin ulcers in rats, Peru balsam was bactericidal, accelerated the repair processes and promoted the differentiation of scar tissue. The promotion of granulation tissue was demonstrated in rat studies with a 5 to 15 % paraffinic solution of Peru balsam administered topically (Hansel, 1993).
3.1.2. Secondary pharmacodynamics
No data available.
3.1.3. Safety pharmacology
3.1.4. Pharmacodynamic interactions
No data available.
3.1.5. Conclusions
The scientific literature contains few reports on non-clinical pharmacological studies of Peru balsam. Peru balsam has been reported to show antibacterial properties and wound healing properties in animal studies.
3.2. Overview of available pharmacokinetic data regarding the herbal substance(s), herbal preparation(s) and relevant constituents thereof
No pharmacokinetic data on the herbal substance is available.
The percutaneous absorption of benzyl benzoate and benzyl alcohol was determined in vivo in monkeys. Absorption through occluded skin was high for both compounds (approximately 70 % of the applied dose in 24 hours). Under non-occlusive conditions, skin penetration of the substances was reduced, presumably because of variations in the rates of evaporation from the site of application (Bronaugh et al., 1990).
3.3. Overview of available toxicological data regarding the herbal substance(s)/herbal preparation(s) and constituents thereof
3.3.1. Single dose toxicity
The acute oral LD50 of Peru balsam in rats was reported as >5g/kg b.w. and the acute dermal LD50 in rabbits reported as >10 g/kg b.w. (Opdyke, 1974).
3.3.2. Repeat dose toxicity
No data available for the herbal substance.
3.3.3. Genotoxicity
No data available for the herbal substance.
3.3.4. Carcinogenicity
No data available for the herbal substance.
3.3.5. Reproductive and developmental toxicity
No data available for the herbal substance.
3.3.6. Local tolerance
Undiluted Peru balsam applied to intact or abraded rabbit skin for 24 hours under occlusion was moderately irritating (Opdyke, 1974).
Studies conducted by Research Institute for Fragrance Materials Inc. (RIFM) (unpublished data) showed that crude Peru balsam is a more potent sensitizer than either the oil or the absolute in the murine local lymph node assay (Api, 2006).
Ulker et al. have studied the sensitising potential and the irritation properties of Peru balsam by combining the local lymph node assay ex vivo (local lymph node assay-5-bromo-2'-deoxyuridine, LLNA-BrdU) and an irritancy assay in vivo. Peru balsam, at concentrations of 0, 0.5, 5, 25, and 50 % (w/v) in acetone: olive oil (4:1, v/v), was applied daily for 3 consecutive days to the dorsum of both ears of female BALB/c mice. Peru balsam was reported to show a moderate sensitising potential with an EC3 value (the estimated concentration necessary to produce a 3-fold increase in proliferation in draining lymph nodes) of 3.09 %. The results of the irritancy assay were expressed as percentage change in ear swelling. The reported percentage of change in ear swelling observed after exposure to a concentration of 5, 25 and 50 % of Peru balsam were 7 %, 15 % and 30 %, respectively (Ulker et al., 2014).
3.3.7. Other special studies
No data available for the herbal substance.
3.3.8. Conclusions
Toxicological studies on Peru balsam are limited. Data on genotoxicity, carcinogenicity, reproductive and developmental toxicology of Peru balsam have not been reported. In a murine local lymph node assay, undiluted Peru balsam showed sensitising potential.
3.4. Overall conclusions on non-clinical data
The scientific literature contains few reports on non-clinical pharmacological studies of Peru balsam. Peru balsam has been reported to exhibit antibacterial properties and wound healing properties in animal studies.
Specific data on pharmacokinetics of the herbal substance is not available. In an in vivo study in monkeys, the absorption of benzyl benzoate through occluded skin was high, but under non-occlusive conditions the absorption was reduced.
Non-clinical information on the safety of Peru balsam is limited. In a murine local lymph node assay, Peru balsam showed sensitising potential. No data on genotoxicity, carcinogenicity, reproductive and developmental toxicology of Peru balsam have been reported. As there is no information on reproductive and developmental toxicity, the use during pregnancy and lactation cannot be recommended.
4. Clinical Data
4.1. Clinical pharmacology
4.1.1. Overview of pharmacodynamic data regarding the herbal substance(s)/preparation(s) including data on relevant constituents
No data available for the herbal substance.
4.1.2. Overview of pharmacokinetic data regarding the herbal substance(s)/preparation(s) including data on relevant constituents
Applied to healthy human skin at a concentration of 25 % in vaseline, Peru balsam was still detectable on the skin after 1 hour using an IR spectroscopic method, but no longer after 8 hours (Hansel, 1993).
The constituents benzyl benzoate and benzyl cinnamate are rapidly hydrolysed to benzyl alcohol, which is oxidised to benzoic acid. Benzoic acid is conjugated in the liver with glycine to hippuric acid and rapidly excreted via the kidneys. About 75-80 % of benzoic acid was eliminated in 6 hours in humans after oral administration (Hansel, 1993).
4.2. Clinical efficacy
4.2.1. Dose response studies
No data available.
4.2.2. Clinical studies (case studies and clinical trials)
No data available.
4.3. Clinical studies in special populations (e.g. elderly and children)
No data available.
4.4. Overall conclusions on clinical pharmacology and efficacy
There are insufficient data on clinical pharmacology or efficacy available for Peru balsam to support a well-established use indication.
5. Clinical Safety/Pharmacovigilance
5.1. Overview of toxicological/safety data from clinical trials in humans
No data available.
5.2. Patient exposure
There is no information on patient exposure to Peru balsam from clinical trials. Most information on human exposure is from the use of Peru balsam as a food or cosmetic ingredient.
Oral use
Peru balsam is used in beverages, ices, candy, baked products and desserts and is classified by the Council of Europe as a natural source of flavourings category 2, i.e. plants, animals and other organisms, and parts of these or products thereof, and preparations derived from, not normally consumed as food items, herbs or species in Europe. These source materials and preparations, on the basis of the information available, are not considered to constitute a risk to health in the quantities used (Council of Europe, 2000).
Peru balsam is approved for food use (Generally Recognized as Safe, GRAS) by the FDA (Leung, 1996).
A group ADI of 0-5 mg/kg b.w. for benzoic acid, the benzoate salts, benzaldehyde, benzyl acetate, benzyl alcohol and benzyl benzoate, expressed as benzoic acid equivalent, was established by JECFA (Joint Food and Agriculture Organization of the United Nations, FAO/WHO Expert Committee on Food Additives) in 1996 (Tisserand and Young, 2014).
Cutaneous use
In 1982 the International Fragrance Association (IFRA) banned the use of crude Peru balsam in fragrance and since then, the crude Peru balsam has not been used in perfumery (Api, 2006). According to the current IFRA standard on Peru balsam crude (exudation of Myroxylon pereirae), the recommendation is prohibited use as a fragrance ingredient due to the risk of sensitization. The IFRA
Standards form the basis for the globally accepted and recognized risk management system for the safe use of fragrance ingredients and are part of the IFRA Code of Practice. This is the self-regulating system of the industry, based on risk assessments carried out by an independent Expert Panel (webpage of IFRA http://www.ifraorg.org).
The only forms of Peru balsam used in perfumery are the extracts or distillates. Peru balsam used in perfumery is prepared either by vacuum distillation, i.e. Peru balsam oil, or by solvent extraction, i.e. Peru balsam absolute. The current IFRA Standard restricts the use of all extracts and distillates of Peru balsam to a total level of 0.4 % in consumer products. This limit has been in effect since 1991 (Api, 2006). The conclusion in the standard is based on the No Expected Sensitization Induction Level (NESIL) established by the Research Institute for Fragrance Materials (RIFM) expert panel. The NESIL of Peru balsam extracts and distillates is 950 mikrog/cm2 (IFRA Standard on Peru balsam extracts and distillates, 2009).
A GC-MS analysis of a sample of Peru balsam oil tested by RIFM shows that the material contains 58.2 % benzyl benzoate, 13.5 % (trans)-cinnamic acid, 12.4 % (trans+ cis)-benzyl cinnamate, 8.1 % benzoic acid, 4.2 % (trans)-nerolidol, 1.2 % benzyl alcohol and 0.7 % (trans)-methyl cinnamate. An estimate of the concentration of the volatiles in Peru balsam absolute is as follows: 49.5 % benzyl benzoate, 11.5 % (trans)-cinnamic acid, 10.5 % (trans+cis)-benzyl cinnamate, 6.9 % benzoic acid,
3.6 % (trans)-nerolidol, 1.0 % benzyl alcohol and 0.6 % (trans)-methyl cinnamate. Cinnamic aldehylde is not a constituent of Peru balsam oil or Peru balsam absolute (Api, 2006).
According to the European Commission database 'Cosing', which provides information on cosmetic substances and ingredients (contained in the Cosmetics Regulation EC No 1223/2009, Cosmetics Directive 76/768/EEC and Inventory of Cosmetic Ingredients), Peru balsam is included in Annex II, i.e. the list of substances prohibited in cosmetic products, and should not be used as a fragrance. The prohibition relates to the crude exudates only. Peru balsam extracts and distillates may be used as a fragrance ingredient of cosmetic products up to a maximum concentration of 0.4 % in the finished cosmetic product. The recommendation is based on a wide variety of test results on the sensitizing potential of Peru balsam (SCCP/0988/06).
5.3. Adverse events, serious adverse events and deaths
Sensitisation
Hjort stated in his thesis on "Eczematous allergy to balsams. Allied perfumes and flavouring agents -with special reference to balsam of Peru" published in 1961 that allergic reactions to Peru balsam have been known since 1880. In the thesis, a case report of Mogling's in 1880 is quoted as being the first description of dermatitis from balsam of Peru. Also Lewin (1881), an American monograph by Morrow (1887) and the French Dermatological Society by Julien (1899) are quoted and they all have described skin reactions from Peru balsam (Hjort, 1961).
Hjort concluded in his thesis that from studies of Blumenthal and Jaffé (1933), Zündel (1936), Engellhardt (1935) and Bonnevie (1939) it became generally appreciated that Peru balsam was a common cause of eczema. Bonnevie, examining 1065 subjects among Danish patients with eczema, found 5.6 % with a positive reaction to Peru balsam. In the 60's, according to Hjort, the majority of European dermatological clinics used routine patch test series that include Peru balsam. At that time, the frequency of positive reactions by routine patch tests in various European clinics varied between 0.4-7 % (Hjort 1961).
In his thesis, Hjort stated that Peru balsam is obsolete from a medical point of view and its use is limited, as its allergenic properties have been well-known in Denmark since 1939. However, Hjort reported that the incidence of positive reactions to Peru balsam had remained at a high level, varying between approximately 2 and 4 % of all patients examined (in total 7500 patients). Half of the patients who admitted contact with Peru balsam had used it for burns. Hjort anticipated that it is generally accepted that sensitisations are particularly liable to occur during the treatment of burns (Hjort 1961).
Allergic reactions to Peru balsam and its constituents have been described in several handbooks and scientific literature, and Peru balsam is considered a contact allergen. For example, it is stated that Peru balsam is one of the most common contact allergens, and dermatitis as a result of contact with the balsam has been well documented (Leung, 1996). Adverse reactions described are contact urticaria and other skin symptoms, recurrent aphthous mouth ulcers, Quincke-Edema, broncho-obstructive and anaphylactic reactions and vasculitis in the form of diffuse purpurea. The constituents of Peru balsam, that are responsible for the sensitisation, are not known (Hansel, 1993).
In the Commission E monograph, Peru balsam is contraindicated for distinct allergic dispositions. Allergic skin reactions are described as side effects. It should not be used for more than one week (Blumenthal, 1990). Continued application of Peru balsam to the skin may cause sensitisation (Martindale, 1972).
In a patch test study at six Scandinavian clinics, 6.9 % of 5558 patients showed positive reactions to Peru balsam (Magnusson et al., 1968).
Baer et al., reported the most common contact allergens of the patient population of the New York University Skin and Cancer unit during the years 1968 to 1979. Out of 340 patients tested, 7.9 % showed a positive reaction to Peru balsam (25 % in petrolatum) (Baer et al., 1973).
From 1995 to 1998, 2273 patients at the Dermatologisches Zentrum in Buxtehude, Germany, with contact dermatitis were tested with the standard series (patch testing), which include Peru balsam and 445 (19.6 %) of the patients showed positive reaction to Peru balsam. The author cited that Peru balsam is the third most common contact allergen in Switzerland, Germany and Austria (Hausen,
2001).
In the Technical Dossier on Dermal Sensitization Quantitative Risk Assessment (QRA) For Fragrance Ingredients in 2006 (published at the IFRA webpage), it is described that the Research Institute for Fragrance Materials (RIFM) sponsored a survey of the patch test database at the Contact Allergy Unit, University Hospital Leuven, Belgium. In this survey a total of 3323 subjects were investigated between 2000 and 2005. Of these patients, 6.7 % were found to have a positive patch test reaction to Peru balsam.
Between January 1990 and December 2005, 10128 patients underwent patch testing at the Dermatology department in Leuven and 6 % reacted positively to Peru balsam (Nardelli et al., 2008).
In a report from Germany, 59 334 patients were tested for contact allergy to Peru balsam (25 % in petrolatum) between 1996 and 2002. The result for positive reaction varied from 7.3 % to 11.5 % (see figure 1 below) (Schnuch et al., 2004).
MyroxyLon pereirae
|
n |
% |
% |
% |
% |
% |
% |
RI |
FR |
|
tested |
0) |
<+) |
<++-) |
(+-4-) |
(irntint i |
(positive) | ||
|
L.4 |
U.6 |
0.4 |
7.3 |
0.3 | ||||
|
B776 |
,’.:i |
;.,4 |
2.0 |
O.i. |
03 |
9 |
0.5 |
71 |
|
8807 |
19 |
7.1. |
2.3 |
0.7 |
0.4 |
L0.6 |
0.5 |
"1 .7 |
|
8792 |
3.1 |
7.Ï |
IS) |
0.7 |
0.7 |
L 1.5 |
0.5 |
61L |
|
8652 |
3.8 |
7.7 |
L.9 |
O.n |
0.6 |
L0 |
0.4 |
74.9 |
|
mu |
".4 |
2.2 |
0.7 |
0.4 |
L0.3 |
0.5 |
"1 .> | |
|
8139 |
3.-F |
n.i. |
2.1 |
0.6 |
0.4 |
13 |
0.4 |
71.5 |
Figure 1: The result for positive reactions in patients tested for contact allergy to Peru balsam (25 % in petrolatum) in a study in Germany between 1996 and 2002 (Schnuch et al., 2004)
In the Scientific Committee on Consumer Safety (SCCS) Opinion on Fragrance allergens in cosmetic products in 2011, numerous results from fragrance contact allergy screening have been reported. The results for Peru balsam (25 % petrolatum) is presented in figure 2 below. Due to the high number of positive patch test reports on Peru balsam, the SCCS considered Peru balsam to pose a high risk of sensitisation to the consumer (SCCS/1459/11).
|
Sweden [36) |
Consecutive patients |
2000 |
3790 |
6.5 |
|
Nine European countries [50) |
Consecutive patients |
2002-2003 |
9672 |
6.1 |
|
Germany, three Swiss and one Austrian Dept. (41) |
Consecutive patients |
2005-20 D8 |
36919 |
B.O (7.7-3.3) |
|
Ten depts. From seven ELI countries (63) |
Consecutive patients |
1996-2DD0 |
26210 |
6.0 |
|
USA [Canada)[61) |
Probably consecutive patients |
2003 |
1603 |
6.6 |
|
NACDG 2009 (62) |
Consecutive patients |
2005-2006 |
4449 |
11,9 |
|
Country (Ref.) |
Population |
ïear(s) |
No, tested |
Crude % positive {05% C3)f |
|
Tel Aviv, Israel (56} |
Consecutiva patients |
1999-20DO |
943 |
6.6 [5.1-3,4)s |
|
South Korea (53) |
Consecutiva patients |
04/2002 -06/2003 |
422 |
7.3 (5.1-10.3)* |
|
Tel Aviv, Israel (57) |
Consecutive patients |
1993-2004 |
2156 |
3.6 [2.9-4.5)* |
|
Man ¡pai, India (55) |
Dermatitis patients |
1939-1998 |
17B0 |
1.0 [0.5 - 1.5)* |
|
Tehran, Iran (5B) |
Consecutive patients |
2002-2004 |
250 |
2,4 [0.9-5.2)* |
|
Sevilla, Spain (74) |
Consecutive patients |
2002-20 D4 |
363 |
5.3 [4.3-7,5)* |
|
Ankara, Turkey (59) |
Consecutiva patie nts |
1992-20D4 |
103B |
2.1 [1.3-3.2)* |
|
Vienna, Austria (16) |
Consecutive patients of cne di nie |
1997-20DO |
266 D |
5.4 [4.6-6.3)* |
|
Czech Republic (38) |
Consecutiva patients |
1997-2001 |
12058 |
7.3 [6.8-7.3)* |
|
Copenhagen, Denmari. (49) |
Consecutive patients |
1935-2007 |
16173 |
3.9 [3.6—4.2)* |
Figure 2: Positive patch test reports on Peru balsam cited in the Scientific Committee on Consumer Safety Opinion on Fragrance allergens in cosmetic products in 2011 (SCCS/1459/11)
|
Country (Kef.) |
Population |
Year(s) |
Ftaqmce allergen |
No, tested |
% positive (05% CI]! |
|
Thailand (91) |
Convenience samplle {via advertisement), age IE-55 |
Not given |
Isoeuqenol, Evemia pm nastri, Myroxylon pereirae * |
2545 |
Positive to at least one of three allergens: 2.5 (1.9-3.2)1 |
|
■Germany (7B) |
Subgroup of MONICA sample, age 25-74 |
1904/05 |
Myroxylon pereirae |
1141 |
2.4 |
|
Denmark |
Population sample, age 1S-69 |
1990 2006 |
Myroxylon pereirae |
567 3460 |
1.1 0.1 |
Figure 3: Results from patch testing with Peru balsam in different population based groups cited in the Scientific Committee on Consumer Safety Opinion on Fragrance allergens in cosmetic products in 2011 (SCCS/1459/11)
The study in Denmark, cited in the figure 3 above, is further presented in the figure 4 below.
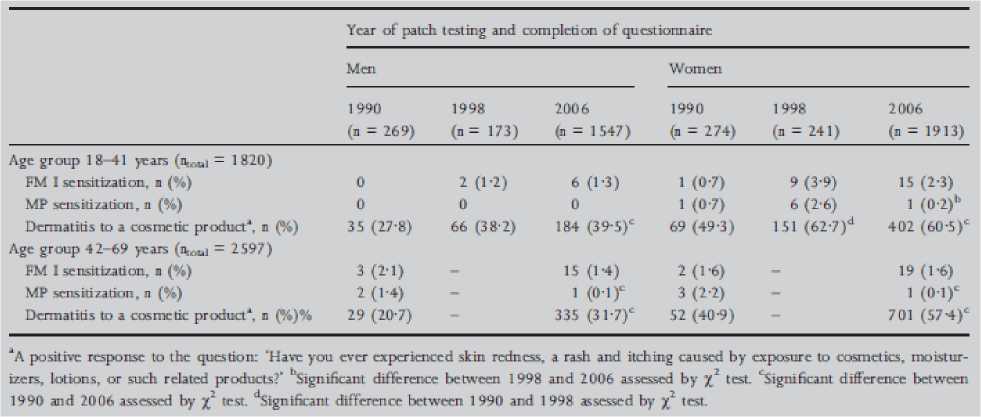
Figure 4: The prevalence of patch test sensitivity to fragrance mix (FM) I and Myroxylon pereirae (MP) and the frequency of self-reported dermatitis to a cosmetic product among men and women aged 18-41 years and 42-69 years in three repeated cross-sectional studies performed in the same general population in Copenhagen, Denmark (1990, 1998 and 2006) (Thyssen et al., 2009)
Photosensitisation
In a photo patch-test, Peru balsam was able to induce photosensitisation in 0.7 and 3 % of two groups of 1129 and 1993 patients, respectively (Hansel, 1993).
Pharmacovigilance databases
In the EudraVigilance database for the period up to October 2014, there were no reports of suspected adverse drug reactions associated with Peru balsam as a single active ingredient.
In the Vigisearch database of the World Health Organization's Uppsala Monitoring Centre for the period up to October 2014, there were several spontaneous reports of suspected adverse drug reactions associated with the single-ingredient Myroxylon balsamum var. pereirae (rout of administration not specified). Most of the reports are associated with allergic reactions and the highest numbers of reports
are found for the preferred terms "dermatitis allergic" (20), "dermatitis contact" (31) and "eczema" (41) (see table 4).
Table 4: Spontaneous reports of suspected adverse-drug reactions from the Vigisearch database of the World Health Organization's Uppsala Monitoring Centre
|
Preferred term (PT) |
Number of reports |
|
Angioedema |
2 |
|
Application site reaction |
1 |
|
Arthritis |
1 |
|
Cheilitis |
1 |
|
Dermatitis |
1 |
|
Dermatitis allergic |
20 |
|
Dermatitis atopic |
1 |
|
Dermatitis contact |
31 |
|
Dermatitis bullous |
3 |
|
Dyspnoea |
1 |
|
Eczema |
41 |
|
Erythema |
1 |
|
Face oedema |
1 |
|
Hypersensitivity |
3 |
|
Lacrimal disorder |
1 |
|
Laryngospasm |
1 |
|
Photosensitivity reaction |
2 |
|
Pruritus |
4 |
|
Rash |
3 |
|
Rash erythematous |
2 |
|
Rash maculo-papular |
1 |
|
Rash popular |
1 |
|
Rhinitis allergic |
1 |
|
Skin necrosis |
1 |
|
Skin ulcer |
1 |
|
Urticaria |
1 |
5.4. Laboratory findings
No data available.
5.5. Safety in special populations and situations
5.5.1. Use in children and adolescents
The use in children under 18 years of age has not been established due to lack of adequate data.
5.5.2. Contraindications
Hypersensitivity to Peru balsam or its constituents (esters of cinnamic and benzoic acids, especially benzyl cinnamate, cinnamyl cinnamate and benzyl benzoate).
5.5.3. Special Warnings and precautions for use
During the treatment, intense UV-exposure of the respective skin areas should be avoided due to risk of photosensitisation.
The use in children and adolescents under 18 years of age has not been established due to lack of adequate data.
If the symptoms worsen during the use of the medicinal product or if signs of skin infection are observed, a doctor or a qualified health care practitioner should be consulted.
5.5.4. Drug interactions and other forms of interaction
No data available.
5.5.5. Fertility, pregnancy and lactation
There are no data on the use of Peru balsam in medicinal products during pregnancy and lactation in humans. The use of Peru balsam should not be recommended during pregnancy and lactation due to insufficient data. No data on fertility is available.
5.5.6. Overdose
5.5.7. Effects on ability to drive or operate machinery or impairment of mental ability
5.5.8. Safety in other special situations
No data available.
5.6. Overall conclusions on clinical safety
There is no safety data on Peru balsam from clinical trials In the literature, Peru balsam is considered one of the most common contact allergens and adverse reactions described in medicinal handbooks are for example dermatitis, contact urticaria, Quincke-Edema, broncho-obstructive and anaphylactic reactions.
Most of the reported frequencies of contact allergy to Peru balsam derive from patients patch tested for suspected allergic contact dermatitis and cannot be related to the general population. However, as stated in the Scientific Committee on Consumer Safety (SCCS) Opinion on Fragrance allergens in cosmetic products, these data are useful in identifying trends and persisting problems. In patch test studies reported in the scientific literature, prevalence figures of positive reactions to Peru balsam of up to 19.6 % have been reported. Importantly, in studies in the general population prevalence figures of positive reaction to Peru balsam of up to 2.6 % have been reported. Furthermore, skin sensitisation and allergic reactions have been reported to the Vigisearch database and the highest numbers of reports are found for the preferred terms "dermatitis allergic", "dermatitis contact" and "eczema".
The use of crude Peru balsam as a fragrance has been banded by the International Fragrance Association (IFRA) since 1982. In addition, in the Cosmetics Regulation EC No 1223/2009, Cosmetics Directive 76/768/EEC and Inventory of Cosmetic Ingredients, Peru balsam (exudate) is included in Annex II, i.e. the list of substances prohibited in cosmetic products, and should not be used as a fragrance. The recommendation is based on a wide variety of test results on the sensitising potential of Peru balsam.
Due to the high risk of sensitisation it is concluded that safer medicinal products and other treatment options are available in the proposed indication. In addition, the medicinal use of Peru balsam in EU today is limited and crude Peru balsam is banned as fragrance in cosmetics in the EU. Therefore, the requirement laid down in Article 16a(1)(e) of Directive 2001/83/EC that "the data on the traditional use of the medicinal product are sufficient; in particular the product proves not to be harmful in the specified conditions of use and the pharmacological effects or efficacy of the medicinal product are plausible on the basis of long-standing use and experience" is not considered fulfilled.
6. Overall conclusions (benefit-risk assessment)
There is no documentation available for Peru balsam to support a well-established use indication. The traditional medicinal use of Peru balsam has been documented in several medicinal handbooks throughout a period of at least 30 years, including at least 15 years within the EU. However, due to the high risk of sensitisation and since there are other treatment options available in the proposed indication, the medicinal use of Peru balsam in EU today is limited.
In the literature, Peru balsam is considered one of the most common contact allergens and adverse reactions described in medicinal handbooks are for example dermatitis, contact urticaria, Quincke-Edema, broncho-obstructive and anaphylactic reactions. In patch test studies reported in the scientific literature, prevalence figures of positive reactions to Peru balsam of up to 19.6 % have been reported. Importantly, in studies in the general population prevalence figures of positive reaction to Peru balsam of up to 2.6 % have been reported. Furthermore, skin sensitisation and allergic reactions have also been reported to the Vigisearch database and the highest numbers of reports are found for the preferred terms "dermatitis allergic", "dermatitis contact" and "eczema". In addition, in the Cosmetics Regulation EC No 1223/2009, Cosmetics Directive 76/768/EEC and Inventory of Cosmetic Ingredients, Peru balsam (exudate) is included in Annex II, i.e. the list of substances prohibited in cosmetic
products, and should not be used as a fragrance. The recommendation is based on a wide variety of test results on the sensitising potential of Peru balsam.
Non-clinical information on the safety of Peru balsam is limited. In a murine local lymph node assay, Peru balsam showed sensitising potential. No data on genotoxicity, carcinogenicity, reproductive and developmental toxicology of Peru balsam have been reported.
Based on these extensive safety concerns, the establishment of an EU herbal monograph on Myroxyion balsamum var. pereirae (Royle) Harms, balsamum, cannot be recommended. The requirement laid down in Article 16a(1)(e) of Directive 2001/83/EC that "the data on the traditional use of the medicinal product are sufficient; in particular the product proves not to be harmful in the specified conditions of use and the pharmacological effects or efficacy of the medicinal product are plausible on the basis of long-standing use and experience" is not considered fulfilled. Safer medicinal products and other treatment options are available in the proposed indication.
Annex
List of references
Page 18/18
Assessment report on Myroxyion balsamum (L.) Harms var. pereirae (Royle) Harms,
balsamum
EMA/HMPC/712648/2014