Vistabel 4 Allergan Units/0.1Ml Powder For Solution For Injection
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
VISTABEL, 4 Allergan Units/0.1 ml, Powder for solution for injection
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Botulinum toxin type A1 .....................4 Allergan Units per 0.1 ml of
reconstituted solution.
1of Clostridium botulinum
Allergan Units are not interchangeable with other preparations of botulinum toxin. Vial of 50 units.
For a full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Powder for solution for injection.
White Powder
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
When the severity of the following facial lines has an important psychological impact in adult patients, VISTABEL is indicated for the temporary improvement in the appearance of:
• moderate to severe vertical lines between the eyebrows seen at maximum frown (glabellar lines),
• moderate to severe lateral canthal lines (crow’s feet lines) seen at maximum smile,
• moderate to severe crow’s feet lines seen at maximum smile and glabellar lines seen at maximum frown when treated simultaneously.
4.2 Posology and method of administration
Posology
Refer to specific recommendations for each indication described below.
Botulinum toxin units are not interchangeable from one product to another. Doses recommended in Allergan Units are different from other botulinum toxin preparations.
Elderly _ patients
There is limited phase 3 clinical data with VISTABEL in patients older than 65 years (see section 5.1).
No specific dose adjustment is required for use in the elderly.
Paediatric population
The safety and effectiveness of VISTABEL in the treatment of glabellar lines seen at maximum frown or crow’s feet lines seen at maximum smile in individuals under 18 years of age have not been demonstrated. The use of VISTABEL is not recommended in individuals under 18 years (see section 4.4).
Method of Administration
VISTABEL should only be administered by physicians with appropriate qualifications and expertise in this treatment and use of the required equipment.
VISTABEL, after reconstitution, must be used only for one session of injection(s) per patient. The excess of unused product must be disposed of as detailed in section 6.6. Particular precautions should be taken for product preparation and administration as well as for the inactivation and disposal of the remaining unused solution (see section 4.4 and 6.6).
The recommended injection volume per muscle site is 0.1 ml. See also dilution table in section 6.6.
For instructions for use, handling and disposal of the vials, see section 6.6.
Care should be taken to ensure that VISTABEL is not injected into a blood vessel when it is injected in the vertical lines between the eyebrows seen at maximum frown (also known as glabellar lines) or in the lateral canthal lines seen at maximum smile (also known as crow’s feet lines), see section 4.4.
Administration Instructions _ for Glabellar Lines seen at maximum _ frown:
Reconstituted VISTABEL (50 Units/1.25 ml) is injected using a sterile 30 gauge needle. 0.1 ml (4 Units) is administered in each of the 5 injection sites (see Figure 1): 2 injections in each corrugator muscle and 1 injection in the procerus muscle for a total dose of 20 Units.
Before injection, the thumb or index finger is to be placed firmly below the orbital rim in order to prevent extravasation below the orbital rim. The needle should be oriented superiorly and medially during the injection. In order to reduce the risk of eyelid ptosis, the maximum dose of 4 Units for each injection site as well as the number of injection sites should not be exceeded. In addition, injections near the levator palpebrae superioris muscle must be avoided, particularly in patients with larger brow-depressor complexes (depressor supercilii). Injections in the corrugator muscle must be done in the central part of that muscle, a distance of at least 1 cm above the arch of the eyebrows.
Figure 1:
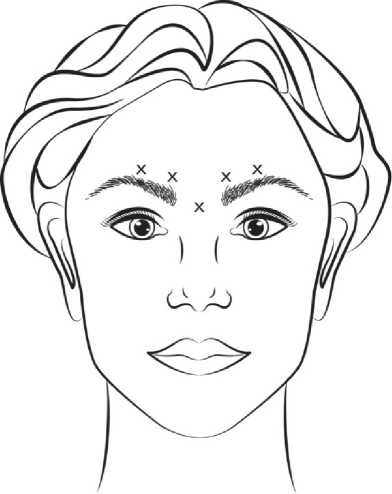
Improvement of severity of glabellar lines seen at maximum frown generally occurs within one week after treatment. The effect was demonstrated for up to 4 months after injection.
Treatment intervals should not be more frequent than every three months. In the event of treatment failure or diminished effect following repeat injections, alternative treatment methods should be employed.
Administration Instructions for Crow’s Feet Lines seen at maximum smile:
Reconstituted VISTABEL (50 Units/1.25 ml) is injected using a sterile 30 gauge needle. 0.1 ml (4 Units) is administered in each of the 3 injection sites per side (total of 6 injection sites) in the lateral orbicularis oculi muscle, for a total dose of 24 Units in a total volume of 0.6 ml (12 Units per side).
In order to reduce the risk of eyelid ptosis, the maximum dose of 4 Units for each injection site as well as the number of injection sites should not be exceeded. In addition, injections should be made temporal to the orbital rim, thereby maintaining a safe distance from the muscle controlling eyelid elevation.
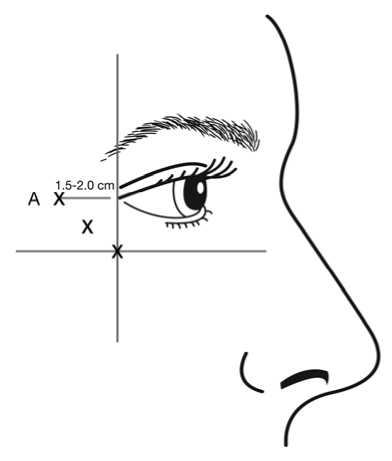
Injections should be given with the needle tip bevel up and oriented away from the eye. The first injection (A) should be made approximately 1.5 to 2.0 cm temporal to the lateral canthus and just temporal to the orbital rim. If the lines in the crow’s feet region are above and below the lateral canthus, inject as shown in Figure 2. Alternatively, if the lines in the crow’s feet region are primarily below the lateral canthus, inject as shown in Figure 3.
Figure 2:
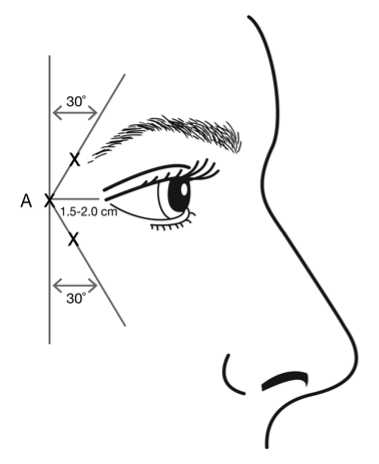
Figure 3:
For simultaneous treatment with glabellar lines seen at maximum frown, the dose is 24 Units for crow’s feet lines seen at maximum smile and 20 Units for glabellar lines (see Administration Instructions for Glabellar Lines, and Figure 1), for a total dose of 44 Units in a total volume of 1.1 ml.
Improvement of severity of crow’s feet lines seen at maximum smile, when assessed by the investigator, occurred within one week of treatment. The effect was demonstrated for a median of 4 months after injection.
Treatment intervals should not be more frequent than every 3 months.
General information
In case of treatment failure after the first treatment session, i.e. in the absence, at one month after injection, of significant improvement from baseline, the following approaches may be considered:
• Analysis of the causes of failure, e.g. incorrect muscles injected, injection technique, formation of toxin-neutralising antibodies, insufficient dose;
• Re-evaluation of the relevance of treatment with botulinum toxin type A;
In the absence of any undesirable effects secondary to the first treatment session, initiate a second treatment session with at least a three-month interval between the two treatment sessions.
For glabellar lines seen at maximum frown, in case of insufficient dose, initiate a second treatment session by adjusting the total dose up to 40 or 50 Units, taking into account the analysis of the previous treatment failure.
The efficacy and safety of repeat injections of VISTABEL beyond 12 months has not been evaluated.
4.3 Contraindications
VISTABEL is contraindicated,
• In individuals with a known hypersensitivity to botulinum toxin type A or to any of the excipients of the formulation;
• In the presence of myasthenia gravis or Eaton Lambert Syndrome
• In the presence of infection at the proposed injection sites.
4.4 Special warnings and precautions for use
Particular precautions should be taken for product preparation and administration as well as for the inactivation and disposal of the remaining unused solution (see sections 4.2 and 6.6).
This medicinal product contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially “sodium free”.
The relevant anatomy, and any alterations to the anatomy due to prior surgical procedures, must be understood prior to administering VISTABEL and injection into vulnerable anatomic structures must be avoided.
The recommended dosage and frequency of administration of VISTABEL should not be exceeded.
An anaphylactic reaction may occur very rarely after injection of botulinum toxin. Epinephrine (adrenaline) or any other anti-anaphylactic measures should therefore be available.
Patients with unrecognised neuromuscular disorders may be at increased risk of clinically significant systemic effects including severe dysphagia and respiratory compromise from typical doses of botulinum toxin type A. In some of these cases, dysphagia has lasted several months and required placement of a gastric feeding tube (see section 4.3).
Caution should also be exercised when VISTABEL is used for treatment of patients with amyotrophic lateral sclerosis or with peripheral neuromuscular disorders.
Adverse reactions possibly related to the spread of toxin distant from the site of administration have been reported very rarely with botulinum toxin (see section 4.8). Patients treated with therapeutic doses may experience exaggerated muscle weakness. Swallowing and breathing difficulties are serious and can result in death. Injection of VISTABEL is not recommended in patients with a history of dysphagia and aspiration.
Patients or caregivers should be advised to seek immediate medical care if swallowing, speech or respiratory disorders arise.
Too frequent or excessive dosing may enhance the risk of antibody formation. Antibody formation may lead to treatment failure of botulinum toxin type A even for other indications.
As is expected for any injection procedure, localised pain, inflammation, paraesthesia, hypoaesthesia, tenderness, swelling/oedema, erythema, localised infection, bleeding and/or bruising have been associated with the injection. Needle-related pain and/or anxiety have resulted in vasovagal responses, including transient symptomatic hypotension and syncope.
Caution should be taken when VISTABEL is used in the presence of inflammation at the proposed injection site(s) or when the targeted muscle shows excessive weakness or atrophy.
Care should be taken to ensure that VISTABEL is not injected into a blood vessel when it is injected in the glabellar lines seen at maximum frown or in the crow’s feet lines seen at maximum smile, see section 4.2.
There is a risk of eyelid ptosis following treatment, refer to Section 4.2 for administration instructions on how to minimise this risk.
The use of VISTABEL is not recommended in individuals under 18 years. There is limited phase 3 clinical data with VISTABEL in patients older than 65 years.
4.5 Interaction with other medicinal products and other forms of interaction
Theoretically, the effect of botulinum toxin may be potentiated by aminoglycoside antibiotics, spectinomycin, or other medicinal products that interfere with neuromuscular transmission (e.g. neuromuscular blocking agents).
The effect of administering different botulinum neurotoxin serotypes at the same time or within several months of each other is unknown. Excessive neuromuscular weakness may be exacerbated by administration of another botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin.
No specific tests have been carried out to establish the possibility of clinical interaction with other medicinal products. No other interactions of clinical significance have been reported in this indication.
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no adequate data from the use of botulinum toxin type A in pregnant women. Studies in animals have shown reproductive toxicity (see section 5.3). The potential risk for humans is unknown. VISTABEL is not recommended during pregnancy and in women of childbearing potential not using contraception.
Breast-feeding
There is no information on whether VISTABEL is excreted in human milk. The use of VISTABEL during breast-feeding cannot be recommended.
Fertility
There are no adequate data on the effects on fertility from the use of botulinum toxin type A in women of childbearing potential. Studies in male and female rats have shown fertility reductions (see section 5.3).
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. However, VISTABEL may cause asthenia, muscle weakness, dizziness and visual disturbance, which could affect driving and the operation of machinery.
4.8 Undesirable effects
a) General
In controlled clinical trials for glabellar lines seen at maximum frown, adverse events considered by the investigators to be related to VISTABEL were reported in 23.5% (placebo: 19.2%) of patients. In treatment cycle 1 of the pivotal controlled clinical trials for crow’s feet lines seen at maximum smile, such events were reported in 7.6% (24 Units for crow’s feet lines alone) and 6.2% (44 Units: 24 Units for crow’s feet lines administered simultaneously with 20 Units for glabellar lines) of patients compared to 4.5% for placebo. Adverse reactions may be related to treatment, injection technique or both. In general, adverse reactions occur within the first few days following injection and are transient. Most adverse events reported were of mild to moderate severity.
The expected pharmacological action of botulinum toxin is a local muscle weakness. However, weakness of adjacent muscles and/or muscles remote from the site of injection has been reported. Blepharoptosis, which may be technique-related, is consistent with the pharmacological action of VISTABEL. As is expected for any injection procedure, pain/burning/stinging, oedema and/or bruising may be observed in association with the injection. Fever and flu syndrome have also been reported after injections of botulinum toxin.
b) Adverse reactions - frequency
The adverse reactions are classified by System Organ Class and frequency defined as follows: Very Common (> 1/10); Common (> 1/100, <1/10); Uncommon (> 1/1,000, <1/100); Rare (> 1/10,000, <1/1,000); Very Rare (<1/10,000).
Glabellar Lines
|
System Organ Class |
Preferred Term |
Frequency |
|
Infections and infestations |
Infection |
Uncommon |
|
Psychiatric disorders |
Anxiety |
Uncommon |
|
Nervous system disorders |
Headache, paraesthesia |
Common |
|
Dizziness |
Uncommon | |
|
Eye disorders |
Eyelid ptosis |
Common |
|
Blepharitis, eye pain, visual disturbance (includes vision blurred) |
Uncommon | |
|
Gastrointestinal disorders |
Nausea |
Common |
|
Oral dryness |
Uncommon | |
|
Skin and subcutaneous tissue disorders |
Erythema, skin tightness |
Common |
|
Oedema (face, eyelid, periorbital), photosensitivity reaction, pruritus, dry skin |
Uncommon |
|
Musculoskeletal and connective tissue disorders |
Localised muscle weakness |
Common |
|
Muscle twitching |
Uncommon | |
|
General disorders and administration site conditions |
Face pain, injection site oedema, ecchymosis, injection site pain, injection site irritation |
Common |
|
Flu syndrome, asthenia, fever |
Uncommon |
Crow’s Feet Lines
The following adverse drug reactions were reported in the double-blind, placebo-controlled clinical studies following injection of VISTABEL 24 Units for crow’s feet lines alone:
|
System organ class |
Preferred term |
Frequency |
|
Eye disorders |
Eyelid oedema |
Common |
|
General disorders and administration site conditions |
Injection site haemorrhage*, injection site haematoma* |
Common |
|
Injection site pain*, injection site paraesthesia |
Uncommon |
*procedure-related adverse reactions
Crow’s Feet Lines and Glabellar Lines
The following adverse drug reactions were reported in double-blind, placebo-controlled clinical studies following injection of VISTABEL 44 Units (simultaneous treatment of crow’s feet lines and glabellar lines):
|
System organ class |
Preferred term |
Frequency |
|
General disorders and administration site conditions |
Injection site haematoma* |
Common |
|
Injection site haemorrhage*, injection site pain* |
Uncommon |
*procedure-related adverse reactions
No change was observed in the overall safety profile following repeat dosing.
c) Post-Marketing data (frequency not known)
The following adverse reactions or medically relevant adverse events have been reported since the drug has been marketed for the treatment of glabellar lines, crow’s feet lines and other clinical indications:
|
System organ class |
Preferred term |
|
Immune system disorders |
Anaphylaxis, angioedema, serum sickness, urticaria |
|
Metabolism and nutrition disorders |
Anorexia |
|
Nervous system disorders |
Brachial plexopathy, dysphonia, dysarthria, facial paresis, hypoaesthesia, muscle weakness, myasthenia gravis, peripheral neuropathy, paraesthesia, radiculopathy, syncope, facial palsy |
|
Eye disorders |
Angle-closure glaucoma (for treatment of blepharospasm), lagophthalmos, strabismus, vision blurred, visual disturbance |
|
Ear and labyrinth disorders |
Hypoacusis, tinnitus, vertigo |
|
Respiratory, thoracic and mediastinal disorders |
Aspiration pneumonia, dyspnoea, bronchospasm, respiratory depression, respiratory failure |
|
Gastrointestinal disorders |
Abdominal pain, diarrhoea, dry mouth, dysphagia, nausea, vomiting |
|
Skin and subcutaneous tissue disorders |
Alopecia, dermatitis psoriasiform, erythema multiforme, hyperhidrosis, madarosis, pruritus, rash |
|
Musculoskeletal and connective tissue disorders |
Muscle atrophy, myalgia |
|
General disorders and administration site conditions |
Denervation atrophy, malaise, pyrexia |
Adverse reactions possibly related to the spread of toxin distant from the site of administration have been reported very rarely with botulinum toxin (e.g. muscle weakness, dysphagia, constipation or aspiration pneumonia which can be fatal) (see section 4.4).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard.
4.9 Overdose
Overdose of VISTABEL is a relative term and depends upon dose, site of injection, and underlying tissue properties. No cases of systemic toxicity resulting from accidental injection of botulinum toxin type A have been observed. Excessive doses may produce local, or distant, generalised and profound neuromuscular paralysis. No cases of ingestion of botulinum toxin type A have been reported.
Signs of overdose are not apparent immediately post-injection. Should accidental injection or ingestion occur, the patient should be medically supervised for several days for signs and symptoms of general weakness or muscle paralysis. Admission to hospital should be considered in patients presenting symptoms of botulinum toxin type A poisoning (generalised weakness, ptosis, diplopia, swallowing and speech disorders, or paresis of the respiratory muscles).
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Muscle relaxants, peripherally acting agents, ATC code: M03A X01.
Botulinum toxin type A (Clostridium botulinum neurotoxin) blocks peripheral acetylcholine release at presynaptic cholinergic nerve terminals by cleaving SNAP-25, a protein integral to the successful docking and release of acetylcholine from vesicles situated within the nerve endings leading to denervation of the muscle and therefore to a paralysis.
After injection, there is an initial rapid high-affinity binding of toxin to specific cell surface receptors. This is followed by transfer of the toxin across the plasma membrane by receptor-mediated endocytosis. Finally, the toxin is released into the cytosol. This latter process is accompanied by progressive inhibition of acetylcholine release, clinical signs are manifest within 2-3 days, with peak effect seen within 56 weeks of injection.
Recovery after intramuscular injection takes place normally within 12 weeks of injection as nerve terminals sprout and reconnect with the endplates.
Clinical data:
Glabellar Lines
537 patients with moderate to severe glabellar lines seen at maximum frown have been included in clinical studies.
VISTABEL injections significantly reduced the severity of glabellar lines seen at maximum frown for up to 4 months, as measured by the investigator assessment of glabellar line severity at maximum frown and by subject’s global assessment of change in appearance of his/her glabellar lines seen at maximum frown. None of the clinical endpoints included an objective evaluation of the psychological impact.
Thirty days after injection 80% (325/405) of VISTABEL-treated patients were considered by investigators as treatment responders (none or mild severity at maximum frown), compared to 3% (4/132) of placebo-treated patients. At this same timepoint, 89% (362/405) of VISTABEL-treated patients felt they had a moderate or better improvement, compared to 7% (9/132) of placebo-treated patients.
VISTABEL injections also significantly reduced the severity of glabellar lines at rest. Of the 537 patients enrolled, 39% (210/537) had moderate to severe glabellar lines at rest (15% had no lines at rest). Of these, 74% (119/161) of VISTABEL-treated patients were considered treatment responders (none or mild severity) thirty days after injection, compared with 20% (10/49) of placebo-treated patients.
There is limited phase 3 clinical data with VISTABEL in patients older than 65 years. Only 6.0% (32/537) of subjects were >65 years old and efficacy results obtained were lower in this population.
Crow’s Feet Lines
1362 patients with moderate to severe crow’s feet lines seen at maximum smile, either alone (N=445, Study 191622-098) or also with moderate to severe glabellar lines seen at maximum frown (N=917, Study 191622-099), were enrolled.
VISTABEL injections significantly reduced the severity of crow’s feet lines seen at maximum smile compared to placebo at all timepoints (p <0.001) for up to 5 months. This was measured by the proportion of patients achieving a crow’s feet lines severity rating of none or mild at maximum smile in both pivotal studies; until day 150 (end of study) in Study 191622-098 and day 120 (end of first treatment cycle in Study 191622-099). For both investigator and subject assessments, the proportion of subjects achieving none or mild crow’s feet lines severity seen at maximum smile was greater in patients with moderate crow’s feet lines seen at maximum smile at baseline, compared to patients with severe crow’s feet lines seen at maximum smile at baseline. Table 1 summarises results at day 30, the timepoint of the primary efficacy endpoint.
In Study 191622-104 (extension to Study 191622-099), 101 patients previously randomised to placebo were enrolled to receive their first treatment at the 44 Units dose. Patients treated with VISTABEL had a statistically significant benefit in the primary efficacy endpoint compared to placebo at day 30 following their first active treatment. The response rate was similar to the 44 Units group at day 30 following first treatment in Study 191622-099. A total of 123 patients recieved 4 cycles of 44 Units VISTABEL for combined crow’s feet and glabellar lines treatment.
Table 1. Day 30: Investigator and Patient Assessment of Crow’s Feet Lines Seen at Maximum Smile
- Responder Rates (% of Patients Achieving Crow’s Feet Lines Severity Rating of None or Mild)
|
Clinical Study |
Dose |
VISTABEL |
Placebo |
VISTABEL |
Placebo |
|
Investigator Assessment |
Patient Assessment | ||||
|
191622-098 |
24 Units (crow’s feet lines) |
66.7%* (148/222) |
6.7% (15/223) |
58.1%* (129/222) |
5.4% (12/223) |
|
191622-099 |
24 Units (crow’s feet lines) |
54.9%* (168/306) |
3.3% (10/306) |
45.8%* (140/306) |
3.3% (10/306) |
|
44 Units (24 Units crow’s feet lines; 20 Units glabellar lines) |
59.0%* (180/305) |
3.3% (10/306) |
48.5%* (148/305) |
3.3% (10/306) | |
*p < 0.001 (VISTABEL vs placebo)
Improvements from baseline in subject assessment of the appearance of crow’s feet lines at maximum smile were seen for VISTABEL (24 Units and 44 Units) compared to placebo, at day 30 and at all timepoints following each treatment cycle in both pivotal studies (p < 0.001).
Treatment with VISTABEL 24 Units also significantly reduced the severity of crow’s feet lines at rest. Of the 528 patients treated, 63% (330/528) had moderate to severe crow’s feet lines at rest at baseline. Of these, 58% (192/330) of VISTABEL-treated patients were considered treatment responders (none or mild severity) thirty days after injection, compared with 11% (39/352) of placebo-treated patients.
Improvements in subjects’ self-assessment of age and attractiveness were also seen for VISTABEL (24 Units and 44 Units) compared to placebo using the Facial Line Outcomes (FLO-11) questionnaire at the primary timepoint of day 30 (p<0.001) and at all subsequent timepoints in both pivotal studies.
In the pivotal studies, 3.9% (53/1362) of patients were older than 65 years of age. Patients in this age group had a treatment response as assessed by the investigator, of 36% (at day 30) for VISTABEL (24 Units and 44 Units). When analysed by age groups of <50 years and >50 years, both populations demonstrated statistically significant improvements compared to placebo. Treatment response for VISTABEL 24 Units, as assessed by the investigator, was lower in the group of subjects >50 years of age than those <50 years of age (42.0% and 71.2%, respectively).
Overall VISTABEL treatment response for crow’s feet lines seen at maximum smile is lower (60%) than that observed with treatment for glabellar lines seen at maximum frown (80%).
916 patients (517 patients at 24 Units and 399 patients at 44 Units) treated with VISTABEL had specimens analysed for antibody formation. No patients developed the presence of neutralising antibodies.
5.2 Pharmacokinetic properties
a) General characteristics of the active substance:
Distribution studies in rats indicate slow muscular diffusion of 125I-botulinum neurotoxin A complex in the gastrocnemius muscle after injection, followed by rapid systemic metabolism and urinary excretion. The amount of radiolabeled material in the muscle declined with a half-life of approximately 10 hours. At the injection site, the radioactivity was bound to large protein molecules, whereas in the plasma it was bound to small molecules, suggesting rapid systemic metabolism of the substrate. Within 24 hours of dosing, 60% of the radioactivity was excreted in the urine. Toxin is probably metabolised by proteases and the molecular components recycled through normal metabolic pathways.
Classical absorption, distribution, biotransformation and elimination (ADME) studies on the active substance have not been performed due to the nature of this product.
b) Characteristics in patients:
It is believed that at therapeutic doses, low systemic distribution of VISTABEL occurs. Clinical studies using single fibre electromyographic techniques have shown increased electrophysiologic neuromuscular activity in muscles distant to the injection site, with no associated clinical signs or symptoms.
5.3 Preclinical safety data
In reproductive studies in mice, rats, and rabbits, embryo toxicity was observed with high doses (delayed ossification and reduced foetal bodyweight). No teratogenic effects were observed in these species. In rats adverse effects on male fertility and female estrous cycling and fertility occurred only at high doses.
Studies on acute toxicity, repeated dose toxicity, local tolerance, mutagenicity, antigenicity and blood compatibility did not show unusual adverse local or systemic effects at clinically relevant dose levels.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Human albumin
Sodium chloride
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
6.3 Shelf life
3 years.
After reconstitution, immediate use of the solution is recommended; however, physicochemical stability for 24 hours between 2°C - 8°C has been demonstrated.
6.4 Special precautions for storage
Store in a refrigerator (2°C - 8°C).
For storage conditions of the reconstituted medicinal product, see section 6.3.
6.5 Nature and contents of container
Powder in a vial (Type I glass) fitted with a stopper (chlorobutyl rubber) and a seal (aluminium);
Vial of 50 Allergan Units of Botulinum toxin type A - pack of one or pack of two
NOT ALL PACK SIZES MAY BE MARKETED
6.6 Special precautions for disposal
Reconstitution should be performed in accordance with good practices rules, particularly for the respect of asepsis. VISTABEL has to be reconstituted with a 0.9% preservative free sodium chloride solution for injection. As per the dilution table below, the requested amount of sodium chloride 9 mg/ml (0.9%) solution for injection has to be drawn up into a syringe in order to obtain a reconstituted solution at a concentration of 4 Units/0.1 ml;
|
added |
(Units per 0.1 ml) |
|
(0.9% sodium | |
|
chloride solution) to | |
|
a 50 Units vial | |
|
1.25 ml |
4.0 Units |
The central part of the rubber cap has to be cleaned with alcohol.
To avoid VISTABEL denaturation, the solution is prepared by injecting the solvent slowly into the vial and by gently rotating the vial avoiding bubble formation. The vial has to be discarded if the vacuum does not pull the solvent into the vial. Once reconstituted, the solution should be visually inspected prior to use. Only clear, colourless to slightly yellow solution without particles should be used.
It is mandatory that VISTABEL is used for one single patient treatment only during a single session.
Procedure to follow for a safe disposal of vials, syringes and materials used:
Immediately after use, and prior to disposal, unused reconstituted VISTABEL solution in the vial and/or the syringe must be inactivated, with 2 ml of dilute sodium hypochlorite solution at 0.5% or 1% and should be disposed of in accordance with local requirements.
Used vials, syringes, and materials should not be emptied and must be discarded into appropriate containers and disposed of as a Medical Biohazardous Waste in accordance with local requirements.
Recommendations in the event of an accident when handling botulinum toxin.
In the event of an accident when handling the product, whether in the vacuum-dried state or reconstituted, the appropriate measures described below must be initiated immediately.
• The toxin is very sensitive to heat and certain chemical agents
• Any spillage must be wiped up: either with an absorbent material soaked in a solution of sodium hypochlorite (Javel solution) in the case of the vacuum-dried product, or with a dry absorbent material in the case of the reconstituted product.
• Contaminated surfaces must be cleaned with an absorbent material _soaked in a solution of sodium hypochlorite (Javel solution) and then
dried.
• If a vial is broken, carefully collect up the pieces of glass and wipe up the product as stated above, avoiding cutting the skin.
• If splashed, wash with a solution of sodium hypochlorite and then rinse thoroughly with plenty of water.
• If splashed into the eyes, rinse one’s eyes thoroughly with plenty of water or with an eye wash solution.
• If the operator injures himself (cuts, pricks himself), proceed as above and take the appropriate medical steps according to the dose injected.
This instruction for use and handling, and disposal should be strictly followed.
7 MARKETING AUTHORISATION HOLDER
ALLERGAN PHARMACEUTICALS IRELAND
Castlebar Road
Westport
County Mayo
Ireland
8 MARKETING AUTHORISATION NUMBER(S)
PL 41443/0010
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
16/05/2008
10 DATE OF REVISION OF THE TEXT
19/03/2015