Acetazolamide 500Mg Powder For Solution For Injection
Out of date information, search another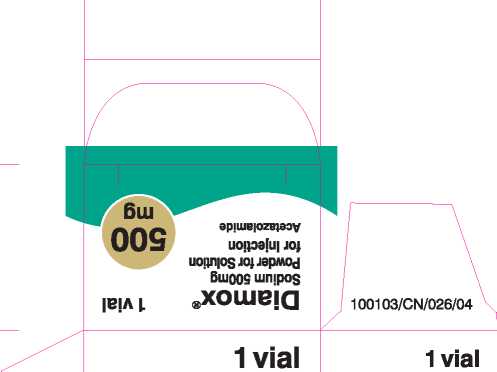
Each vial contains: Acetazolamide 500mg (as Acetazolamide sodium).
Also contains sodium hydroxide and hydrochloric acid.
Acetazolamide
Sodium 500mg Powder for Solution for Injection Acetazolamide
For IV or IM use |
See leaflet lor further information. Use as directed by the doctor.
Keep out of the sight and reach of children.
Do not store above 25°C.
Reconstitute with at least 5ml of Water for Injection prior to use. For single use only.
Any unused solution can be stored in a refrigerator for up to 24 hours but any unused solution after this period must be discarded.
PL 12762/0146 I POM I
LOT
EXP
Marketing Authorisation Holder: Mercury Pharmaceuticals Ltd., Capital House, 85 King Wiiliam Street, London EC4N 7BL, UK
Mercury Pharma Group Ltd. is licensed to use the registered trademark Diamox
02
69
165378


/
f \
|
MercuryPharma | |
|
Version No.: |
100103/CN/026/04 |
|
Product Name: |
Diamox Sodium 500mg Powder for Sol. for Inj. |
|
Pack Size: |
1 vial |
|
Component: |
Carton |
|
SKU: |
100103 |
|
Market: |
UK |
|
Production Site: |
BAG |
|
Revision No.: |
2 |
|
Revision Date: |
05/02/2015 |
|
Revised by: |
PAT |
V_/
|
A | |
|
Dimension: |
40 x 70 x 30mm |
|
Commodity No.: |
N/A |
|
Pharma Code: |
N/A |
|
Core Spec Ref: |
N/A |
|
DCMF: |
N/A |
|
Print Colours: |
334 C, 4515 C& Black |
|
Non-Print Colours: |
Cutler |
|
Tech App. Date: |
No |
|
Min. Font Size: |
7 pt |
|
V |
J |
|
r |
A |
|
CRF: |
AMCo.CRF.133.2013 |
|
DOA: |
N/A |
|
DOI: |
N/A |
|
V |
J |
f A
REGULATORY AUTHORITY APPROVAL CONFIRMATION
Confirmation that this artwork has been approved by the appropriate market authority (if applicable, e.g. MHRA, HPRA, etc.) and that Mercury Pharma have license approval to distribute this component for sale in the relevant market.
Accept Artwork / Reject Artwork
(Please strike off whichever NOT applicable)
Signature
Name
Date ...........................................................................
\_/
r \
|
€ MercuryPharma | |
|
Version No.: |
100103/LB/026/04 |
|
Product Name: |
Diamox Sodium SOOmg Powder for Sol. for Inj. |
|
Pack Size: |
1 vial |
|
Component: |
Label |
|
SKU: |
100103 |
|
Market: |
UK |
|
Production Site: |
BAG |
|
Revision No.: |
2 |
|
Revision Date: |
05/02/2015 |
|
Revised by: |
PAT |
\_____/
|
r | |
|
Dimension: |
76 x 30mm |
|
Commodity No.: |
N/A |
|
Pharma Code: |
N/A |
|
Core Spec Ref: |
N/A |
|
DCMF: |
N/A |
|
Print Colours: |
334 C, 4515 C&Black |
|
Non-Print Colours: |
Cutler |
|
Tech App. Date: |
No |
|
Min. Font Size: |
4 pt |
|
V |
J |
1 vial
Diamox®
Sodium SOOmg Powder for Solution for Injection
Acetazolamlde

al House.
85 Khg William Street, London EC4N 7BL, UK
PL12762/0146 IPOH | 100103/LB/026/04
Use as ejected by the doctor.
Keep out of the sight and reach of children.
Do not store above 25°C.
Reconstitute with at least 5ml of Water for Injection pnorto use. For single use only.
Any unused solution can be stored in a refrigerator for up to
|
r |
A |
|
CRF: |
AM Co.CRF.133.2013 |
|
DOA: |
N/A |
|
DOI: |
N/A |
|
V |
J |
( A
REGULATORY AUTHORITY APPROVAL CONFIRMATION
Confirmation that this artwork has been approved by the appropriate market authority (if applicable, e.g. MHRA, HPRA, etc.) and that Mercury Pharma have license approval to distribute this component for sale in the relevant market.
Signature
Name
Date ...........................................................................
\_y
PAGE 1 OF 1