Acetylcysteine 200 Mg/Ml Injection
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Acetylcysteine 200 mg/ml Injection
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Acetylcysteine 200 mg per ml. Each 10ml ampoule contains 2g acetylcysteine.
For excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Concentrate for solution for infusion.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Acetylcysteine is indicated for the treatment of paracetamol overdosage in patients:
a) who have taken a staggered overdose irrespective of plasma paracetamol level. Staggered is defined as an overdose where the paracetamol was ingested over a period of 1 hour or more; or
b) where there is any doubt over the time of the overdose, irrespective of plasma paracetamol level; or
c) who present with a plasma paracetamol level on or above a line joining points of 100mg/L at 4h and 15mg/L at 15h (see below nomogram).
4.2
Posology and method of administration
Acetylcysteine should be administered by intravenous infusion preferably using Glucose 5% as the infusion fluid. Sodium Chloride 0.9% solution may be used if Glucose 5% is not suitable.
Adults
The full course of treatment with acetylcysteine comprises 3 consecutive intravenous infusions:
First infusion
Initial loading dose of 150 mg/kg body weight of acetylcysteine is infused in 200mL over 1 hour.
Second infusion
50 mg/kg in 500mL over the next 4 hours.
Third infusion
100 mg/kg in 1 litre over the next 16 hours.
The patient should therefore receive a total of 300 mg/kg over a 21 hour period.
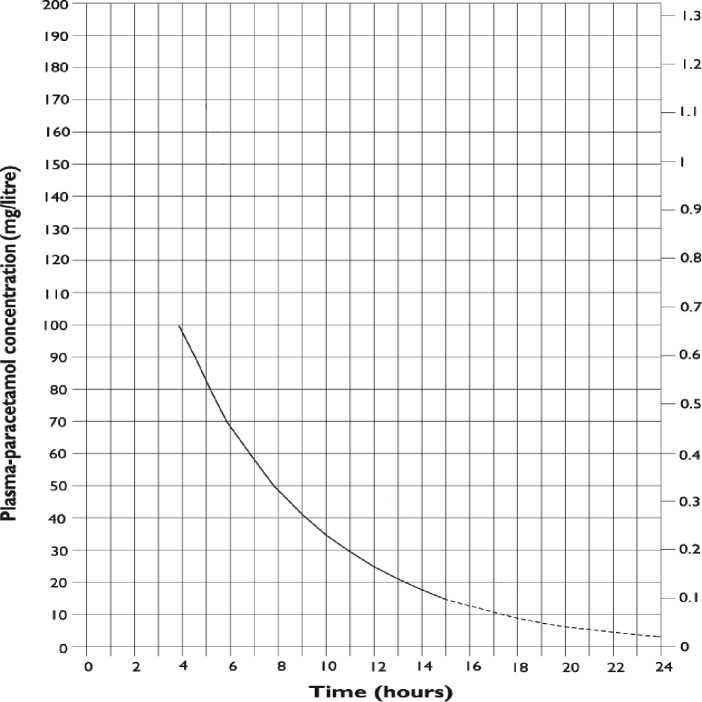
Plasma-paracetamol concentration (mmol/litre)
A ceiling weight of 110 kg should be used when calculating the dosage for obese patients.
Dosage should be calculated using the patient’s actual weight.
|
Adult Acetylcysteine prescription |
Please circle appropriate weight, dose and volume. | |||||||||
|
(each ampoule |
= 200mg/mL Acetylcysteine) | |||||||||
|
Regimen |
Dose 1 |
Dose 2 |
Dose 3 | |||||||
|
Fluid |
200 mLs 5% glucose or sodium chloride 0.9% |
500 mLs 5% glucose or sodium chloride 0.9% |
1000 mLs 5% glucose or sodium chloride 0.9% | |||||||
|
Duration of infusion |
60 minutes |
4 hours |
16 hours | |||||||
|
Drug dose |
150 mg/kg |
50 mg/kg |
100 mg/kg | |||||||
|
Acetylcysteine |
Acetylcysteine |
Acetylcysteine | ||||||||
|
Patient Weight1 |
Dose |
Ampoule volume2 |
Infusion Rate |
Dose |
Ampoule volume2 |
Infusion Rate |
Dose |
Ampoule volume2 |
Infusion Rate | |
|
kg |
mg |
mL |
mL/h |
mg |
mL |
mL/h |
mg |
mL |
mL/h | |
|
40-49 |
6750 |
34 |
234 |
2250 |
12 |
128 |
4500 |
23 |
64 | |
|
50-59 |
8250 |
42 |
242 |
2750 |
14 |
129 |
5500 |
28 |
64 | |
|
60-69 |
9750 |
49 |
249 |
3250 |
17 |
129 |
6500 |
33 |
65 | |
|
70-79 |
11250 |
57 |
257 |
3750 |
19 |
130 |
7500 |
38 |
65 | |
|
80-89 |
12750 |
64 |
264 |
4250 |
22 |
131 |
8500 |
43 |
65 | |
|
90-99 |
14250 |
72 |
272 |
4750 |
24 |
131 |
9500 |
48 |
66 | |
|
100-109 |
15750 |
79 |
279 |
5250 |
27 |
132 |
10500 |
53 |
66 | |
|
>110- Max dose |
16500 |
83 |
283 |
5500 |
28 |
132 |
11000 |
55 |
66 | |
1 Dose calculations are based on the weight in the middle of each band. Ampoule volume has been rounded up to the nearest whole number.
Children
Children should be treated with the same doses and regimen as adults; however the quantity of intravenous fluid should be modified taking into account age and weight, as fluid overload is a potential danger.
Acetylcysteine should be administered by intravenous infusion preferably using Glucose 5% as the infusion fluid. Sodium Chloride 0.9% solution may be used if Glucose 5% is not suitable.
Doses should be administered using an appropriate infusion pump.
The full course of treatment with acetylcysteine comprises 3 consecutive intravenous infusions:
First infusion
Initial loading dose of 150 mg/kg infused over 1 hour (150 mg/kg/h).
Given as a 50 mg/mL solution at a rate of 3 mL/kg/h.
Second Infusion
Dose: 50 mg/kg infused over 4 hours (12.5 mg/kg/h).
Given as a 6.25 mg/mL solution at a rate of 2 mL/kg/h.
Third Infusion
Dose: 100 mg/kg infused over 16 hours (6.25 mg/kg/h).
Given as a 6.25 mg/mL solution at a rate of 1 mL/kg/h.
Preparation of the solution
Dose 1
Prepare a 50 mg/mL solution. Dilute each 10mL ampoule of acetylcysteine (200 mg/mL) with 30 mL glucose 5% or sodium chloride 0.9% to give a total volume of 40 mL.
Dose 2
Prepare a 6.25 mg/mL solution. Dilute each 10 mL ampoule of acetylcysteine (200 mg/mL) with 310 mL glucose 5% or sodium chloride 0.9% to give a total volume of 320 mL.
Dose 3
Prepare a 6.25 mg/mL solution. Dilute each 10 mL ampoule of acetylcysteine (200 mg/mL) with 310 mL glucose 5% or sodium chloride 0.9% to give a total volume of 320 mL.
Any unused solution should be disposed of in accordance with local requirements.
Administration
Weigh the child.
Determine the total volume of solution to be infused (infusion fluid + acetylcysteine prepared as above) into the child from the table. A separate volume will be required for each of the three infusion periods.
For example for a child weighing 12 kg, 38mL of solution is required for Dose 1, 100mL for Dose 2 and 200mL for Dose 3.
Infuse intravenously according to the weight of the child (see table).
For example for a child weighing 12 kg, Dose 1 is infused at 38mL/h over 60 minutes, Dose 2 would be infused at 25mL/h and Dose 3 at 13mL/h.
Doses should be administered sequentially with no break between the doses.
1
|
Paediatric acetylcysteine prescription (each ampoule = 200mg/mL acetylcysteine) |
Please circle appropriate weight, dose and volume. | |||||||||
|
Regimen |
Dose 1 |
Dose 2 |
Dose 3 | |||||||
|
acetylcysteine close |
150 mg/kg |
50 mg/kg |
100 mg/kg | |||||||
|
Duration of infusion |
1 hour |
4 hours |
16 hours | |||||||
|
Infusion concentration |
50mg/ml |
6.25mg/ml |
6.25m g/ml | |||||||
|
Rate of infusion |
3ml/kg/h |
2ml/kg/h |
1ml/kg/h | |||||||
|
Patient Weight1 |
Dose |
Rate of Infusion |
Total Infusion Volume |
Dose |
Rate of Infusion |
Total Infusion Volume |
Dose |
Rate of Infusion |
Total Infusion Volume | |
|
Kg |
mg |
mL/h |
mL |
mg |
mUh |
mL |
mg |
mL/h |
mL | |
|
1 |
150 |
3 |
3 |
50 |
2 |
3 |
100 |
1 |
16 | |
|
2 |
300 |
6 |
6 |
100 |
4 |
16 |
200 |
2 |
32 | |
|
3 |
450 |
9 |
9 |
150 |
6 |
24 |
300 |
3 |
40 | |
|
4 |
600 |
12 |
12 |
200 |
3 |
32 |
400 |
4 |
64 | |
|
5 |
750 |
15 |
15 |
250 |
10 |
40 |
500 |
5 |
80 | |
|
6 |
900 |
18 |
18 |
300 |
12 |
43 |
600 |
8 |
96 | |
|
7 |
1050 |
21 |
21 |
350 |
14 |
56 |
700 |
7 |
112 | |
|
0 |
1200 |
24 |
24 |
400 |
16 |
84 |
300 |
3 |
120 | |
|
9 |
1350 |
27 |
27 |
450 |
10 |
72 |
900 |
9 |
144 | |
|
10-14 |
1875 |
38 |
38 |
825 |
25 |
100 |
1250 |
13 |
200 | |
|
15-19 |
2625 |
53 |
53 |
375 |
35 |
140 |
1750 |
13 |
230 | |
|
20-24 |
3375 |
68 |
68 |
1125 |
45 |
130 |
2250 |
23 |
360 | |
|
25-29 |
4125 |
03 |
03 |
1375 |
55 |
220 |
2750 |
23 |
440 | |
|
30-34 |
4875 |
98 |
98 |
1625 |
85 |
2 B0 |
3250 |
33 |
520 | |
|
35-39 |
5625 |
113 |
113 |
1875 |
75 |
300 |
3750 |
33 |
600 | |
Dose calculations are based on the weight in the middle of each band.
There are no contraindications to the treatment of paracetamol overdose with acetylcysteine.
4.4 Special warnings and precautions for use
Intravenous acetylcysteine, given within 24 hours of ingestion of a potentially hepatotoxic overdose of paracetamol, is indicated to prevent or reduce the severity of liver damage. It is most effective when administered within 8 to 10 hours of a paracetamol overdose. Although the efficacy of acetylcysteine diminishes between 10 and 24 hours post-overdose, it should be administered up to 24 hours as it can still be of benefit. It may still be administered after 24 hours in patients at risk of severe liver damage.
Anaphylactoid reactions
Anaphylactoid hypersensitivity reactions occur with acetylcysteine, particularly with the initial loading dose. The patient should be carefully observed during this period for signs of an anaphylactoid reaction. Nausea, vomiting, flushing, skin rash, pruritus and urticaria are the most common features, but more serious anaphylactoid reactions have been reported where the patient develops angioedema, bronchospasm, respiratory distress, tachycardia and hypotension. In very rare cases these reactions have been fatal. There is some evidence that patients with a history of atopy and asthma may be at increased risk of developing an anaphylactoid reaction.
Most anaphylactoid reactions can be managed by temporarily suspending the acetylcysteine infusion, administering appropriate supportive care and restarting at a lower infusion rate. Once an anaphylactoid reaction is under control, the infusion can normally be restarted at an infusion rate of 50 mg/kg over 4 hours, followed by the final 16 hour infusion (100 mg/kg over 16 hours).
Coagulation
Changes in haemostatic parameters have been observed in association with acetylcysteine treatment, some leading to decreased prothrombin time, but most leading to a small increase in prothrombin time. An isolated increase in prothombin time up to 1.3 at the end of a 21 hour course of acetylcysteine without an elevated transaminase activity do not require further monitoring or treatment with acetylcysteine.
Fluid and electrolytes
Use with caution in children, patients requiring fluid restriction or those who weigh less than <40 kg because of the risk of fluid overload which may result in hyponatraemia and seizures which may be life threatening.
Each 10ml of acetylcysteine for Infusion contains 322.6mg sodium. To be taken into consideration with patients on a controlled sodium diet.
4.5 Interaction with other medicinal products and other forms of interaction
None known.
The safety of acetylcysteine in pregnancy has not been investigated in formal prospective clinical trials. However, clinical experience indicates that use of acetylcysteine for the treatment of paracetamol overdose during pregnancy is effective. Prior to use in pregnancy, the potential risks should be balanced against the potential benefits.
4.7 Effects on ability to drive and use machines
None known.
4.8 Undesirable effects
The most common adverse reactions reported with acetylcysteine are nausea, vomiting, flushing and skin rash.
Less commonly, more serious anaphylactoid reactions have been reported that include angioedema, bronchospasm/respiratory distress, hypotension, tachycardia and hypertension.
Adverse reactions to acetylcysteine usually occur between 15 and 60 minutes after the start of infusion and, in many cases, symptoms are relieved by stopping the infusion. An antihistamine drug may be necessary, and occasionally corticosteroids may be required. Once an adverse reaction is under control, the infusion can normally be restarted at the lowest infusion rate (100mg/kg in 1 litre over 16 hours).
Other reported adverse reactions include: injection site reactions, pruritus, cough, chest tightness or pain, puffy eyes, sweating, malaise, raised temperature, vasodilation, blurred vision, bradycardia, facial or eye pain, syncope, acidosis, thrombocytopenia, respiratory or cardiac arrest, stridor, anxiety, extravasation, arthropathy, arthralgia, deterioration of liver function, generalised seizure, cyanosis, lowered blood urea.
Case reports of fatalities with acetylcysteine have been reported very rarely.
Hypokalaemia and ECG changes have been noted in patients with paracetamol poisoning irrespective of the treatment given. Monitoring of plasma potassium concentration is, therefore, recommended.
If any adverse reactions to acetylcysteine develop, advice should be sought from a National Poisons Centre to ensure that the patient receives adequate treatment of the paracetamol overdose.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
There is a theoretical risk of hepatic encephalopathy. Overdosage of acetylcysteine has been reported to be associated with effects similar to the 'anaphylactic' reactions noted in section 4.8 (‘Undesirable effects’), but they may be more severe. General supportive measures should be carried out. Such reactions are managed with antihistamines and steroids in the usual way. There is no specific antidote.
Death has been reported following massive overdose. A child who received 2,450mg/kg acetylcysteine exhibited reactions including intermittent myoclonus which persisted despite administration of diazepam, lorazepam and phenytoin. Subsequently the patient had irregular breathing, increased frequency of myoclonus and was unresponsive to pain. The post mortem confirmed intracranial hypertension and brain death. The overdose was caused following confusion between the drug and infusion diluent maximum dosages.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Acetylcysteine is believed to reduce the hepatic toxicity of NAPQI (n-acetyl-p-benzo-quinoneimine), the highly reactive intermediate metabolite formed following ingestion of a high dose of paracetamol, by at least two mechanisms. Firstly, acetylcysteine acts as a precursor for the synthesis of glutathione and, therefore, maintains cellular glutathione at a level sufficient to inactivate NAPQI. This is thought to be the main method of action of acetylcysteine in the early stages of paracetamol toxicity.
Acetylcysteine has been shown to still be effective when infusion is started at up to 12 hours after paracetamol ingestion, when most of the analgesic will have been metabolised to its reactive metabolite. At this stage, acetylcysteine is thought to act by reducing oxidised thiol groups in key enzymes.
If acetylcysteine treatment is begun more than 8 to 10 hours after paracetamol overdose, its efficacy in preventing hepatotoxicity (based on serum indicators) declines progressively with further lengthening of the overdose-treatment interval (time between paracetamol overdose and start of treatment). However,
there is now evidence that treatment is still beneficial when given up to 24 hours after overdose. At this late stage of paracetamol hepatotoxicity, acetylcysteine’s beneficial effects may be due to its ability to improve systematic haemodynamics and oxygen transport, although the mechanism by which this may occur has yet to be determined.
5.2 Pharmacokinetic properties
Following intravenous administration of acetylcysteine by the standard 20-hour intravenous regimen, plasma levels of 300 to 900mg/l have been documented to occur shortly after the start of infusion, falling to 11 to 90mg/l at the end of the infusion period. Elimination half-lives from 2 to 6 hours have been reported after intravenous dosing, with 20 to 30% of the administered dose being recovered unchanged in the urine.
Metabolism seems to be rapid and extensive. There is no information on whether acetylcysteine crosses the blood-brain barrier or the placenta, or whether it is excreted in breast milk.
5.3 Preclinical safety data
None stated.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Disodium Edetate Water for Injections Sodium Hydroxide
6.2 Incompatibilities
Acetylcysteine is not compatible with rubber or metals, particularly iron, copper and nickel. Silicone rubber and plastic are satisfactory for use with acetylcysteine.
A change in the colour of the solution to light purple has sometimes been noted and is not thought to indicate significant impairment of safety or efficacy.
6.3 Shelf life
Unopened product: 24 months. Diluted product: use immediately.
6.4 Special precautions for storage
Do not store above 25°C. Keep container in outer carton.
6.5 Nature and contents of container
Clear Type I glass ampoules containing 10ml acetylcysteine 200 mg/ml solution. 10 ampoules are packed per carton.
6.6 Special precautions for disposal
Acetylcysteine solution should be diluted for intravenous infusion using 5% glucose. The volumes to be used are as directed in section 4.2 (‘Posology and Method of Administration’). The solution should be used immediately following dilution, in line with the directions given in section 4.2 (‘Posology and Method of Administration’).
7 MARKETING AUTHORISATION HOLDER
TEVA UK Limited Brampton Road Hampden Park Eastbourne East Sussex BN22 9AG
8 MARKETING AUTHORISATION NUMBER(S)
PL 00289/1543
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
12/12/2008
10 DATE OF REVISION OF THE TEXT
12/08/2015