Actikerall 5 Mg/G + 100 Mg/G Cutaneous Solution
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Actikerall 5 mg/g + 100 mg/g Cutaneous Solution
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
1 g of cutaneous solution contains 5 mg of fluorouracil and 100 mg of salicylic acid.
Excipient with known effect:
80 mg of dimethyl sulfoxide/g of solution.
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Cutaneous solution.
Actikerall is a clear, colourless to slightly orange-white solution.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Actikerall is indicated for the topical treatment of slightly palpable and/or moderately thick hyperkeratotic actinic keratosis (grade I/II) in immunocompetent adult patients.
Grade I/II intensity is based on the 4-point scale of Olsen et al. (1991), see section 5.1.
4.2 Posology and method of administration
Posology in adults including the elderly
Cutaneous use.
Actikerall should be applied to actinic keratoses once daily until the lesions have completely cleared or for up to a maximum of 12 weeks. If severe side effects occur, reduce the frequency of drug application to three times per week until the side effects improve. If areas of skin with a thin epidermis are treated, the solution should be applied less frequently and the course of the therapy monitored more often.
Response can be seen as early as in six weeks. Response increases over time and data are available for treatment up to 12 weeks. Complete healing of the lesion(s) or optimal therapeutic effect may not be evident for up to eight weeks after treatment cessation. When assessing options to treat recurrent lesions, the physician should consider that the efficacy of retreatment with Actikerall has not been formally measured in clinical trials.
Paediatric population
Actinic keratosis is not a disease of a paediatric population. Therefore, the safety and efficacy of Actikerall in this population has not been established.
Method of administration
Multiple actinic keratoses can be treated simultaneously. There is experience in treating up to ten lesions at the same time. The total area of skin being treated with Actikerall at any one time should not exceed 25 cm2 (5 cm x 5 cm).
Actikerall should only come into contact with the actinic keratosis and a rim of max. 0.5 cm of the healthy skin surrounding the lesion.
Actikerall is applied to actinic keratoses by use of the brush applicator connected to the closure cap. To avoid overloading the brush with solution, the brush should be wiped off on the neck of the bottle before application.
The treated area should not be covered after application and the solution should be left to dry to form a film over the applied area.
Each time Actikerall is reapplied the existing film coating should be removed beforehand by simply peeling it off. Warm water may help to remove the film.
Contraindications
4.3
Hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
Actikerall must not be used during the lactation period, an existing pregnancy or by women for whom pregnancy cannot be excluded with certainty.
Actikerall must not be used to treat patients with renal insufficiency.
Actikerall must not be used in conjunction with brivudine, sorivudine and analogues. Brivudine, sorivudine and analogues are potent inhibitors of the fluorouracil-degrading enzyme dihydropyrimidine dehydrogenase (DPD) (see also sections 4.4 and 4.5).
Actikerall must not be allowed to come into contact with the eyes or mucous membranes.
4.4 Special warnings and precautions for use
Actikerall contains the cytostatic agent 5-fluorouracil.
The enzyme dihydropyrimidine dehydrogenase (DPD) plays an important role in the breakdown of fluorouracil. Inhibition, deficiency or decreased activity of this enzyme can result in accumulation of fluorouracil.
If applicable, the determination of DPD enzyme activity is indicated before starting treatment with fluorouracil or other fluoropyrimidines.
Patients who take phenytoin concomitantly with fluorouracil should be regularly tested for elevated plasma levels of phenytoin.
In patients with sensory disturbances (e.g. those with diabetes mellitus) close medical monitoring of the treatment area is required.
Actinic keratosis is due to chronic UV damage and any local irritation where Actikerall has been applied may be made worse by sun exposure. Patients should be counseled to protect the skin against further excessive or cumulative exposure, especially in the area being actively treated.
No experience exists for the treatment of Basal Cell Cancer and Bowen’s disease, which should therefore not be treated with the product.
There is no experience in treating actinic keratoses in an area that is also affected by another skin disease and the clinician should take into account that the outcome of treatment may differ.
There is currently no data available on Actikerall treatment of other body areas apart from the face, forehead and bald scalp.
If areas of skin with a thin epidermis are treated, the solution should be applied less frequently and the course of the therapy monitored more often.
This medicinal product contains dimethyl sulfoxide. May be irritant to the skin.
The bottle should be closed tightly after use or the solution will dry up quickly and can no longer be used correctly.
The solution should not be used if crystals occur.
Actikerall solution should not come into contact with textiles or acrylics (e.g. acrylic bathtubs) as the solution may cause permanent stains.
Caution flammable: keep away from fire or flames.
4.5 Interaction with other medicinal products and other forms of interaction
The enzyme dihydropyrimidine dehydrogenase (DPD) plays an important role in the breakdown of fluorouracil. Nucleoside analogues such as brivudine and sorivudine may lead to a drastic increase in plasma concentrations of fluorouracil or other fluoropyrimidines and thus an associated increase in toxicity. For this reason, an interval of at least 4 weeks between the use of fluorouracil and brivudine, sorivudine and analogues should be observed.
In case of accidental administration of nucleoside analogues such as brivudine and sorivudine to patients who are being treated with fluorouracil, effective measures for reducing fluorouracil toxicity should be taken. Admission to a hospital may be indicated. All necessary measures for protection from systemic infections and dehydration should be introduced.
Elevated plasma levels of phenytoin leading to symptoms of phenytoin intoxication have been reported with the concomitant administration of phenytoin and fluorouracil (see 4.4).
There is no evidence for relevant systemic absorption of salicylic acid, however absorbed salicylic acid may interact with methotrexate and sulphonylureas.
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no data from the use of topical fluorouracil in pregnant women. A teratogenic effect of systemically administered fluorouracil has been observed in animals. Salicylic acid can adversely influence the outcome of pregnancy in rodents.
Breast-feeding
It is unknown whether fluorouracil or its metabolites are excreted in human milk after topical application. A risk to the suckling child cannot be excluded.
Fertility
Fertility studies with systemic fluorouracil resulted in transient male infertility and in reduction of pregnancy rates in female rodents. However, this is unlikely to be of relevance for man, due to the very limited absorption of active compounds after cutaneous administration of Actikerall.
Actikerall is contraindicated in pregnancy and lactation (see section 4.3).
4.7 Effects on ability to drive and use machines
Actikerall has no influence on the ability to drive and use machines.
4.8 Undesirable effects
Adverse reactions according to MedDRA system organ class and in decreasing frequency are listed below. Frequencies are defined as very common (>1/10); common (>1/100 to <1/10); uncommon (>1/1,000 to <1/100); rare (>1/10,000 to <1/1,000); very rare (<1/10,000); and not known (cannot be estimated from the available data).
General disorders and administration site conditions:
Very common: At application site: erythema, inflammation, irritation (including burning), pain, pruritus.
Common: At application site: bleeding, erosion, scab.
Uncommon: At application site: dermatitis, oedema, ulcer.
Skin and subcutaneous tissue disorders:
Common: skin exfoliation.
Nervous system disorders:
Common: headache.
Eye disorders:
Uncommon: dry eye, eye pruritus, increased lacrimation.
Mild to moderate irritation and inflammation at the application site occurred in the majority of patients treated with the solution for actinic keratosis. In case of severe reactions frequency of treatment may be reduced.
As the medicinal product has a very strong softening effect on the stratum corneum, whitish discolorations and scaling of the skin may occur, particularly in the area surrounding the actinic keratosis.
Due to its salicylic acid content, use of this medicinal product may cause slight signs of irritation, such as dermatitis and contact allergic reactions, in patients of a corresponding disposition. Such contact allergy reactions may be manifested in the form of itching, reddening and small blisters even outside the area of application.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellowcard Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
When applied on the skin as recommended, systemic intoxications with either actives is unlikely. Significantly more applications than recommended result
in an increase of frequency of reactions at the application site and their severity.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents; Antimetabolites; Pyrimidine analogues, ATC code: L01BC52
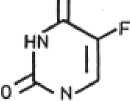
Fluorouracil:
The active substance fluorouracil (FU) is a cytostatic agent that has an antimetabolite effect. Due to its structural similarity with the thymine (5-methyluracil) occurring in nucleic acids, FU prevents its formation and utilisation and in this way inhibits both DNA and RNA synthesis. The result is growth inhibition of those cells in particular which - as in the case of actinic keratosis - are at a stage of accelerated growth and therefore absorb FU in increased quantities. As a consequence also the growth of viruses, which can be involved in actinic keratosis development, is inhibited.
0
H
Fluorouracil
Salicylic acid:
Topical salicylic acid has a keratolytic effect and reduces the hyperkeratosis associated with actinic keratosis. Salicylic acid is considered to be a phenolic aromatic acid and is lipid soluble. Its mechanism of action as a keratolytic and corneolytic agent is thought to be related to its interference with corneocyte adhesion, its solubilising effect on intercellular cement, and its loosening and detachment of corneocytes. By acting as an organic solvent, salicylic acid may remove intercellular lipids covalently linked to the cornified envelope surrounding cornified cells.
In a pivotal randomized, placebo-controlled, double-blind, three-armed, parallel group, multi-center Phase III trial 470 patients with actinic keratosis grade I to II were treated with either a solution of fluorouracil (5 mg/g) and salicylic acid (100 mg/g) (5-FU-SA) or placebo or a diclofenac gel (30 mg/g) (DG). 187 patients were exposed to the fixed combination 5-FU-SA for up to 12 weeks. Primary endpoint was the histological clearance of a lesion 8 weeks post end of treatment. Topical treatment with 5-FU-SA showed superiority to placebo treatment and to DG treatment.
Secondary efficacy endpoints, like total lesion count, total AK lesion size, lesion response, physician’s global assessment and subject’s overall assessment of efficacy confirmed the results of the primary endpoint. In 72.0 % of the subjects in the 5-FU-SA group actinic keratosis could no longer be detected in the biopsy taken, whereas clearance rates in the DG and placebo groups were 59.1 % and 44.8 % respectively (per protocol analysis). The most frequent adverse reactions to 5-FU-SA were application and site irritation (including burning) (86.1 %) and application site inflammation (73.3 %). Also, application site pruritus (44.9 %) and application site pain (25.1%) occurred at a high frequency. Other adverse reactions were site erythema and site erosion. Discontinuation due to skin and application site reactions was low (0.5 %).
There is only clinical experience on the use of Actikerall on the face, forehead and bald scalp. When deciding on treatment of other parts of the body the epidermal thickness in different areas may be taken into consideration:
Average epidermal thickness of different body parts
|
Body part |
Mean thickness (micrometer) |
|
Face |
49.4 |
|
Forehead |
50.3 |
|
Upper trunk front (decollete) |
42.2 |
|
Arms/ legs |
60.1 |
Source: Koehler et al. 2010 (Skin Res Technol 2010; 16:259-264); Sandby-Moller et al. 2003 (Acta Derm Venereol 2003; 83(6):410-3); Whitton et Everall 1973 (Br J Dermatol 1973; 89(5):467-76)
Actinic keratosis lesion intensity was graded according to the 4-point scale based on Olsen et al.,1991 (J Am Acad Dermatol 1991; 24: 738-743):
|
Grade 0 |
none |
Clinical description of intensity grading no AK lesion present, neither visible nor palpable | |
|
I |
mild |
flat, pink maculae without signs of hyperkeratosis |
and |
|
II |
moderate |
erythema, slight palpability, with AK felt better than seen pink to reddish papules and erythematous plaques |
with |
|
III |
severe |
hyperkeratotic surface, moderately thick AK that are easily seen and felt very thick and / or obvious AK | |
5.2 Pharmacokinetic properties
In an absorption study carried out on pigs no fluorouracil was detected in the serum after the cutaneous application - even in large quantities - i.e. the active substance was not absorbed in quantities which could be detected with standard analytical methods (HPLC).
No fluorouracil concentration above 0.05 qg/ml could be identified in actinic keratosis patients (n=12).
According to a pharmacokinetic study analysing the absorption rate of fluorouracil in humans after the application in warts with the same formulation is markedly below 0.1 %.
After application on the skin Actikerall forms a solid film which appears white after the solvent has evaporated. This produces an occlusive effect which promotes penetration of the active substances into the epidermis, where actinic keratoses are located.
Salicylic acid has been added due to its keratolytic properties in order to improve penetration of the active substance, which is particularly difficult in the case of hyperkeratotic actinic keratoses. The same effect is achieved by the excipient dimethyl sulfoxide, which acts as a solubiliser for the active ingredient fluorouracil.
The keratolytic effect of salicylic acid is based on its direct action on the intracellular cement substances or desmosomes, which promote the cornification process.
Experiments on animals and human pharmacokinetic trials have shown that salicylic acid penetrates the surface rapidly, depending on the substrate and other factors influencing penetration, such as the condition of the skin.
Salicylic acid is metabolised by conjugation with glycine to form salicyluric acid, with glucuronic acid on the phenolic OH group to form ether glucuronide and on the COOH group to form ester glucuronide, or by hydroxylation to gentisic acid and dihydroxybenzoic acid. In the normal dose range the half-life of salicylic acid is between 2 and 3 hours, but may increase to 15 to 30 hours in the case of high dosages as a result of the limited capacity of the liver to conjugate salicylic acid.
No toxic side effects are generally to be expected from the topical application of salicylic acid (but see the contraindications), as serum levels above 5 mg/dl are hardly ever reached. Early symptoms of salicylate intoxication can only occur at serum values of more than 30 mg/dl.
5.3 Preclinical safety data
No experimental data on the acute and sub-chronic toxicity of fluorouracil (FU) after topical application are available. In rats dose-dependent systemic bioavailability of
FU occurs and results in severe local reactions and fatal systemic effects due to the antimetabolite actions of FU at such high (up to 10,000 fold above the human) doses that are not reached with Actikerall when used as recommended.
FU was in vitro mutagenic in some test strains. A number of studies investigated carcinogenicity for FU in rodents and showed no effect. However, in a single study there is evidence of carcinogenicity of FU in mice following i.p. administration. Several studies following systemic administration of FU indicate potential high dose teratogenic or embryotoxic effects but less or no effects on fertility or general reproductive performance. Fertility studies with systemic FU resulted in transient male infertility and in reduction of pregnancy rates in female rodents. However, because of the very limited absorption after cutaneous administration, any such effect is very unlikely to be of relevance in man.
Salicylic acid has a low acute toxicity but may induce skin reactions after topical application at higher concentrations. Salicylic acid is not known to have any mutagenic, genotoxic, carcinogenic or teratogenic effects.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Dimethyl sulfoxide Ethanol Ethyl acetate Pyroxyline
Poly(butyl methacrylate, methyl methacrylate)
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
2 years
Shelf life after opening: 3 months
6.4 Special precautions for storage
Do not store above 25 °C.
Do not refrigerate or freeze. Keep the bottle tightly closed.
6.5 Nature and contents of container
This medicinal product is packed in a brown glass bottle with a child resistant closure of white polypropylene in a cardboard carton. The closure of the bottle is connected to a brush to apply the solution. The brush applicator (CE mark) consists of polyethylene (HDPE and LDPE 1:1) with brush hairs of nylon secured in shaft with stainless steel (V2A).
Pack size: 25 ml solution.
6.6 Special precautions for disposal and other handling
Any unused product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Almirall Hermal GmbH Scholtzstrasse 3 21465 Reinbek Germany
8 MARKETING AUTHORISATION NUMBER(S)
PL 33016/0015
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
04/05/2016
10 DATE OF REVISION OF THE TEXT
04/05/2016