Actiq 200 Micrograms Compressed Lozenge With Integral Oromucosal Applicator
Profile Key


PACKAGE LEAFL T: INFORMATION FOR THE USER
ACTIQ® 200 micrograms c impressed lozenge with integral oromuc sal applicator
ACTIQ® 400 micrograms c . impressed lozenge with integral oromuc sal applicator
ACTIQ® 600 micrograms c impressed lozenge with integral oromuc sal applicator
ACTIQ® 800 micrograms c impressed lozenge with integral oromuc sal applicator
-ACTIQ® 1,200 micrograms compressed lozenge with integral oromucosal applicator ACTIQ® 1,600 micrograms c ompressed lozenge with integral orom cosal applicator
Fentanyl
Read all of this leaflet carefull ■ before you ' start using this medicine because it contains important information for you.
Keep thisleaflet. -You -may need to-read-it -again^
If you have any further questions, ask your doctor or pharmacist.
This medicine has been pres ribed for you only. Do not pass it on to oth rs. It may harm them, even if their signs of illness are the same as yours.
If you get any side effects, tali to your doctor or pharmacist. This includes any possible side effects not listed in this leafle'. See section 4.

Page
onoamine-
What is in this leaflet
■ 1. What ACTIQ is and what it is used for
2. What you need to know befoe you use ACTIQ
3. How to use ACTIQ
4. Possible side effects
5. How to store ACTIQ
6. Contents of the pack and othi r information
1. What ACTIQ is and wha1 it is used for
i
ACTIQ contains the active substance fentanyl
■ which-s a strong-pain-retteving medicfneknowm as an opioid. The ACTIQ unit co ies as a lozenge on a stick.
It is used to treat breakthrougi pain in adult patients with cancer who are a lready taking other opioid pain medicines f: r their persistent (around-the-clock) cancer pai ■. Breakthrough pain is additional sudden pain that occurs suddenly in spite of your havi ^ g taken your usual opioid pain-relieving m idicines.
Do not use ACTIQ if you have not been having a prescribed opioid medicine for persistent (around-the-clock) pain regul. rly every day, for at least a week. This is bedause if you are not having such a medicine, using ACTIQ may increase the chances of our breathing becoming dangerously slow o r shallow, or even stopping.
Do not use ACTIQ to treat pain from injuries, surgery, headaches or migraines.
] 2. What you “ need to “know “before you “ use ACTIQ
Do not use ACTIQ:
if you are not regularly using ; prescribed opioid medicine (e.g. codeine, fentanyl, hydromorphone, morphine, ox ycodone, pethidine), every day on a reg> lar schedule, for at least a week, to control youi persistent pain.
If you have not been using th i se medicines you must not use ACTIQ, because it may increase the risk that breathing could become dangerously slow and/or shallow, or even stop. if you are allergic to fentanyl o r any of the other ingredients of this medicine (listed in Section 6).
if you are currently taking ■
oxidase inhibitor (MAOI) medicines for severe depression (or have taken them in the past —-2-weeks}r--
• if you have severe breathing problems or severe lung problems where you ave an obstruction.
• if you suffer from short-tem pain other than breakthrough pain.
Do not use ACTIQ if any of the above apply to you. If you are not sure, talk t your doctor or pharmacist before using ACTiQ.
Warnings and precautions
Keep using the opioid pain medicine you take for your persistent (around-the- lock) cancer pain during your ACTIQ treatment.
Talk to your doctor or pharma cist before using ACTIQ if:
• Your other opioid pain m 'dicine for your persistent (around-the-clock) cancer pain is not stabilised yet.
• You have any illness that affects your breathing (such as asthma, wheezing, or shortness of breath).
• You have a head injury or have had any loss of consciousness.
• You have problems with ybur heart especially slow heart rate.
• You have liver or kidney poblems - this will affect how your system br aks down
the medicine.
• You have low blood press, re due to a low amount of fluid in your circulation.
• You have diabetes.
• You are over 65 years old - you may need a lower dose and any dos i increase will be reviewed very carefully by your doctor.
• You take antidepressants o r antipsychotics, please refer to section using other medicines.
ACTIQ contains approximatel 2 grams of sugar and a frequent consumption exposes you to an increase risk of dental decay that may be serious.
- Thus,- it is-important-to-continue to take - good _ -care of your mouth and teet i during treatment with ACTIQ. If you present su: h serious local effects consult your doctor.
Children and adolescents
ACTIQ is not recommended f r children below 16 years of age.
Other medicines and ACTI i
Do not use this medicine and tell your doctor or pharmacist if you are taking:
• Other fentanyl treatments that have been prescribed for your breakt rough pain in the past. If you still have so me of these fentanyl treatments at ho ^ e, check with your pharmacist how to dispose of them.
Tell your doctor or pharmacist before using ACTIQ if you are taking o' have recently taken or might takeany other medicines. This includes medicines obtained with o ut a prescription, including herbal medicin i s. In particular, tell your doctor or pharmacist if you are taking any of the following medicin is:
• Any medicines which might make you sleepy - such as sleeping pills, medicines to treat anxiety, some medicin’s for allergic reaction (antihistamines), or tranquillisers.
• Some muscle relaxant: - such as baclofen, diazepam.
• _ Any medicines thatmjght affect how your
body breaks down AC > IQ - such as ritonavir or other medicines th t help control HIV infection, other so-calli d 'CYP3A4 inhibitors' such as ketoconazole, itraconazole, or fluconazole (used for f: ngal infections) and troleandomycin, clarithromycin, or erythromycin (medici es for bacterial infections) and so-call d 'CYP3A4 inducers' such as rifampin or rif butin (medicines for bacterial infections), carbamazepine, phenobarbital or phe ytoin (medicines used to treat convulsions/fi :s).
• Certain types of stron i pain killers, called partial agonist/ antagonists e.g. buprenorphine, nalbu. hine and pentazocine (medicines for treatm int of pain). You could experience sym : toms of withdrawal syndrome (nausea, vo ^ iting, diarrhoea, anxiety, chills, tremor, a nd sweating) while using these medicines.
If you are due to have sur ery requiring a general anaesthetic. The isk of side effects increases if you are takin g medicines such as certain antidepressants o: antipsychotics. ACTIQ may interact with these ^ edicines and you may experience mental status changes (e.g. agitation, hallucinations, coma), an ■ other effects such as body temperature above 38 °C, increase in heart rate, unstable blood presure, and exaggeration of reflexes, muscular rigidity, lack of coordination and/or gastrointestinal symptoms (e.g nausea, vomiting, diarrhoea). You' doctor will tell you whether ACTIQ is suitable for you.
ACTIQ with food, drink : nd alcohol
• ACTIQ may be used b ifore or after meals. However do not use du ring meals.
• You may drink some w; ter before using ACTIQ to help moisten your outh. However, do not drink or eat anything hile using ACTIQ.
_ • _ Do -Doi drink grapefruit juice while takin g _ _ ACTIQ. This is because it may affect the way your body breaks down ACTIQ.
• Do not drink alcohol while using ACTIQ. It can increase the chances f getting dangerous side effects.
Pregnancy, breast-feeding and fertility
If you are pregnant or br ast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharma cist for advice before taking this medicine.
You should not use ACTiQ during child-birth because fentanyl may cause breathing difficulties in the new-born child. There is also a risk of the new-born child having withdrawal symptoms of the medicine if ACTIQ is used for a long time
rlurinn nronnann/
Fentanyl can get into breast milk and may cause side effects in the brea st-fed infant. Do not use ACTIQ if you are brea t-feeding. You should not start breast-feeding u: til at least 5 days after the last dose of ACTIQ.
Ask your doctor or ph rmacist for advice before taking any medicine, if you are pregnant or breast-feeding.
I
Driving and using m< chines
This medicine may affect you being able to drive or use any tools or m ichines. Talk to your doctor about whether it is safe for you to drive, or use any tools or machines in the first few hours after taking ACTIQ.
Do not drive or use a y tools or machines if you: feel sleepy or diz: y; have blurred or double vision; have difficulty in concentrating. It is important you know i ow you react to ACTIQ before driving or using any tools or machines. The medicine can aff ct your ability to drive as it may make you sleepy or dizzy.
• Do not drive while taking this medicine until you know how it a ffects you.
• It is an offence to drive if this medicine affects your ability to drive.
However, you would ^ ot be committing an offence if:
l
• The medicine has :een prescribed to treat a medical or dental problem and
• You have taken it according to the instructions given b y the prescriber or in the information provided with the medicine and
• It was not affectin g your ability to drive safely
Talk to your doctor or pharmacist if you are not sure whether it is safe for you to drive while taking this medicine.
ACTIQ contains gluc se and sucrose (types of sugar)
• If you have been t o ld by your doctor that you cannot tolerate or ; igest some sugars, talk to your doctor before using ACTIQ.
• Each lozenge cont ins about 2 grams of glucose. If you have diabetes, you need to take this into acco u nt.
• The glucose in the lozenge may be harmful to the teeth. Always m ake sure you clean your teeth regularly.
3. How to use AC i IQ
Always use this medi cine exactly as your doctor or pharmacist has tol: you. Check with your -dbctbr-br-pharmacist jf-you are-not sure.- -
When you first start using ACTIQ, your doctor will work with you to find the dose that will relieve your breakthrough p in. It is very important that you use ACTIQ exactl ■ as the doctor tells you.
• Do not change dosi s of ACTIQ or your other
pain medicines on .our own. Change in dose must be prescribed and checked by your doctor.
• If you are not sure a bout the right dose or if you have questions about taking this medicine, talk to yo ur doctor.
How the medicine g e ts into your body
When you place the l o zenge in your mouth:
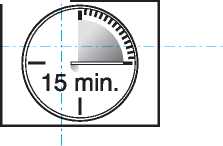
• The lozenge dissoh es and the active substance is release d. it takes around 15 minutes for this to happen.
The active substance is absorbed through the lining of your mouth, into the blood system.
Taking the medicii e like this allows it to be absorbed quickly. This means that it relieves your breakthrough pai i quickly.
While the right d1 se is being found
You should start t : feel some relief quickly while you are taking ACTiQ. However, while you and the -doctor are finding out the dose that controls your breakthrough pai i, you may not get enough pain relief 30 minutes after starting to use one ACTIQ unit (15 minutes fr m when you finish using the ACTIQ unit). If this happens, your doctor may allow you to use a second ACTIQ unit of the same strength for that s a me episode of breakthrough pain. Do not use a second unit unless your doctor tells you to. Never use more than two units to treat a single epis de of breakthrough pain.
While the right dose is being found, you may need to have mor than one strength of ACTIQ units at home. How ever, keep only the strengths of ACTIQ units you need in the house. This is to stop possible con usion or overdose. Talk to your pharmacist about how to dispose of any ACTIQ units you do not eed.
How many units i o use
Once the right dose has been found with your doctor, use 1 unit or an episode of breakthrough pai i .
Talk to your doctor if your right dose of ACTIQ does not relieve y ur breakthrough pain for several episodes f breakthrough pain in a row. _YbULdbctb^wiU decLdeJfyour dose meeds to _ _ be changed.
You must tell your doctor straight away if you are' using ACTIQ more than four times per day. This is because he ma wish to change your medicine for your persistent (around-the-clock) pain. When he has done this, , hen your persistent pain has been controlled, he may need to change your dose of ACTIQ. Fo' the most effective relief, tell your doctor abou' your pain and how ACTIQ is working for you. This is so that the dose can be changed if neede i.
How to use the medicine
Opening the paci - each ACTIQ unit is sealed in
its own blister pack.
• Open the pack when you are ready to use it.
Do not open it in advance.
• Hold the bliste i pack with the printed side away from you.
• Hold the short 1 ab end of the blister pack.
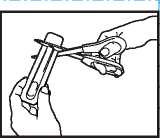
”•" _ Put-scissors-closeTo-the ""
end of ACTIQ unit and cut the long ta ■ end completely off (as shown).
• Separate the printed backing from the blister pack and pull t: e printed backing completely off the blister ack.
• Remove the A TIQ unit from the blister pack and put t i e lozenge in your mouth straight away.
Valid For 7 Days from 24-Oct-2016
ACTIQ PATIENT INFO LFLT GB - 400-33-10321.18
Teva Pharmaceuticals Europe B.V Effective Date: TBD
use ACTIQ until the next breakt
rough
4. Possible side effects
Valid For 7 Days from 24-Oct-2016
Page 2 of 3
Using the ACTIQ unit
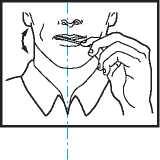
• Put the lozenge between yor cheek and gum.
• Using the handle, keep moving the lozenge round in your mouth, especially along your cheeks. Twirl the handle often.
• To get the most effective reli :f, finish the
lozenge completely _
in 15 minutes.
_ If youjin[sh_too _ quickly, you will swallow more of the medicine and get less relief from your breakthrough pain.
• Do not bite, suck or chew the lozenge. This would mean lower blood levels and less pain relief than when used as ■ irected.
• If for some reason you are not finishing the whole lozenge each time you have breakthrough pain, talk to yo: r doctor.
How often you should use ACTIQ
Once a dose is found that effectively controls your pain, do not use more than four ACTIQ units each day. If you think you ■ ight need to use more than four ACTIQ units per day, talk to your doctor straight awa '.
How many ACTIQ units you shi uld use
Do not use more than two units to treat any sTrigle^pTsodeofbreaRtRrougTi'pain. _
If you use more ACTIQ than you should
The most common side effects f using too much are feeling sleepy, sick or . izzy.
• If you begin to feel dizzy, sick, 1 r very sleepy before the lozenge is complete ly dissolved, take it out of your mouth and c all another person in your house to help ou.
A serious side effect of ACTIQ is : low and/or shallow breathing. This can occ ir if your dose of ACTIQ is too high or if y.u take too much ACTIQ.
• If this happens, get medical help straight away.
What to do if a child or adult a> cidentally takes ACTIQ
If you think someone has accide: tally taken ACTIQ, get medical help straight away. Try to keep the person awake (by calli ng their name an-d-sha-king- their afm-o-r-sh-ou-Fd£f) -until- -emergency help arrives.
If you forget to use ACTIQ
If you still have the breakthroug' pain, you may use ACTIQ as your doctor h: s told you.
If the breakthrough pain has sto ped, do not pain episode.
If you stop using ACTIQ
You should discontinue ACTIQ when you no longer have any breakthrough pain. You must however continue to take . our usual opioid pain relieving medicine t' treat your persistent cancer pain as advise ■ by your doctor. You may experience wit ' drawal symptoms similar to the possibl e side effects of ACTIQ when discontinuing ACTIQ.
If you experience withdrawal symptoms or if
you are concerned about yo' r pain relief you should contact your doctor. Your doctor will evaluate if you need medicine to reduce or eliminate the withdrawal sy ptoms.
If you have any further questions on the use of this medicine, ask your doct or or pharmacist.
Like all medicines, this medicine can have side effects, although not everyb dy gets them. If you notice any of these, contact your doctor. The most serious side effects are shallow
-breathing, low. blood pressurie_and shocks _ _
l
You or your carer should REMOVE the ACTIQ unit from your mout h, contact your doctor immediately and call for emergency help if you exp< rience any of the following serious side i ffects - you may need urgent medical a ttention:
• Becoming very sleepy or I aving slow and/ or shallow breathing.
• Difficulty in breathing or izziness, swelling of the tongue, lip or throa' which may be early signs of serious aller: ic reaction.
Note to Carers:
If you see that the patient taking ACTIQ has slow and/or shallow breathi i g or if you have a hard time waking the perso n up, take the following steps IMMEDIATELY:
• Using the handle, remov e the ACTIQ unit from the person's mouth and keep it out of the reach of children op pets until it is
- -disposed of. —
• CALL FOR EMERGENCY H; LP.
• While waiting for emerge icy help, if the person seems to be breat 'ing slowly, prompt them to breathe e' ery 5-10 seconds.
If you feel excessively dizzy, : leepy or otherwise ill while using ACTIQ, use the handle to remove the lozen g e and dispose of it according to the instructions given in this leaflet (see Section 5). Then contact your doctor for further directions on using ACTIQ.
Very common side effects (affecting more than 1 in 10 people)
• Vomiting, nausea/feeling sick, constipation, stomach (a : dominal) pain
• Asthenia (weakness), sleepiness, dizziness, headaches
• Shortness of breath
I
Common side effects (affectjng 1 to 10 people -in-100) —
• Confusion, anxiety, seeing or hearing things that are not there [hallucinations), depression, mood swing .
• Feeling unwell
• Muscle jerks, feeling of dizziness or "spinning", loss of conscio' sness, sedation, tingling or num b ness, difficulty coordinating movements, increased or altered sensitivity to touc i, convulsions (fits)
• Dry mouth, mouth infla mation, tongue problems (for example, b rning sensation or ulcers), taste alteration
• Wind, abdominal bloatin g, indigestion, decreased appetite, weig't loss
Difficulty passing uri'
• Accidental injury (for example, falls)
Uncommon side effec :s (affecting 1 to 10 people in 1,000)
• Tooth decay, paralysis of the gut, mouth ulcers, gum bleedin g
• Coma, slurred speec'
• Abnormal dreams, f eling detached, abnormal thinking, dxcessive feeling of well being
• Widening of blood v ssels
• Hives
I
Frequency not known
The following side effects have also been reported with the use o'' ACTIQ lozenge but it is not known how often they may occur:
• Receding gums, inflam mation of the gum, tooth loss, severe br athing problems, flushing, feeling very warm, diarrhoea, swelling of arms or legs, fatigue, insomnia, pyrexia, withdrawal s' ndrome (may manifest by the occur rence of the following side effects nausea, vbmiting, diarrhoea, anxiety, chills, tremor, and sweating).
Whilst using ACTIQ you may experience irritation, pain and ulce ■ at the application site and gum bleeding.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This incl u des any possible side effects not listed in this leaflet. You can also report side effects direct ly via the Yellow Card - -Scheme at: www.mhra.gov.uk/yeHowcard-By reporting side effects you can help provide more informati; n on the safety of this medicine.
5. How to store AC! IQ
The pain-relieving me iicine in ACTIQ is very strong and could be lif e -threatening if taken accidentally by a child. ACTIQ must be kept out of the sight and re i ch of children.
• Do not use ACTIQ afer the expiry date shown on the package label and the carton. The expiry d te refers to the last day of that month.
• Do not store above ;0 °C.
• Always keep ACTIQ in its blister package until you are ready t: use it. Do not use if the blister package ias been damaged or opened before you ire ready to use it.
• If you are no longer u sing ACTIQ, or if you ^haveunused ACTIQ pnrtsinyour home, -return all unused pa: ks to your doctor
or pharmacist.
How to dispose of AcTiQ after use
Partially used ACTIQ lozenge may contain enough medicine to be harmful or life-
threatening to a child.
Even if there is a little o^ no medicine left on the handle, the handle itself must be properly disposed of as follows:
• If the medicine is tot lly gone, throw the handle away in a waste container that is out of reach of children and pets.
If any medicine emains on the handle place the lozenge under hot running water to dissolve the remainder and then throw the ' andle away in a waste container that i. out of the reach of children and pets.
• If you do not finish the entire lozenge and you cannot immediately dissolve the remaining medic ine, put the lozenge out of the reach of children and pets until such a time as yi u can dispose of the partially used lo: enge as instructed above.
• Do not flush pa tially used lozenge,
_ handles,_prthe biisteLpackagingdown the toilet.
6. Contents of the pack and other information
What ACTIQ cont ins:
• The active substance is fentanyl.
Each individual lozenge contains either: 200 microgram . fentanyl (as citrate)
400 microgram . fentanyl (as citrate)
600 microgram . fentanyl (as citrate)
800 microgram . fentanyl (as citrate)
1,200 micrograms fentanyl (as citrate)
1,600 microgra - s fentanyl (as citrate)
The other ingre: ients are:
Lozenge:
Dextrates hydrated (equivalent to approximately 2 gr ms of glucose).
Citric acid, disodium phosphate, artificial berry flavour (malto dextrin, propylene -g-iyc-olrartif-icial-fla-vour-s,- an-d-tr-iet-hyl-citrate) magnesium steara te.
Edible glue used to ' ttach the lozenge to the handle
Modified maize ba sed food starch E 1450, confectioner's sug ar (as sucrose and maize starch), water.
Imprinting ink
Water, de-waxed hite shellac, propylene glycol, blue synthetic coal tar dye E 133
What ACTIQ look - like and contents of the pack
ACTIQ consists of a white to off-white solid lozenge attached t a handle for oromucosal application. The lo enge may appear slightly mottled on storag e. This is due to slight changes in the flav. uring agent of the product and does i ot affect how the product works in any way.
ACTIQ exists in 6 different strengths: 200,
400, 600, 800, 1,201 and 1,600 micrograms. The dosage streng h is marked on the white lozenge, on he handle, on the blister package and on th e carton to ensure that you are taking the righ t medicine. Each strength is associated with a specific colour.
ACTIQ lozenges ar e supplied in individual blister packages.
Blister packages are supplied in cartons of 3, 6, 15 or 30 individual ACTIQ units.
Not all pack size may be marketed.