Actiq 800 Micrograms Compressed Lozenge With Integral Oromucosal Applicator
PACKAGE LEAFLET: INFORMATION FOR THE USER
ACTIQ® 200 micrograms compressed lozenge with integral oromucosal applicator ACTIQ® 400 micrograms compressed lozenge with integral oromucosal applicator ACTIQ® 600 micrograms compressed lozenge with integral oromucosal applicator ACTIQ® 800 micrograms compressed lozenge with integral oromucosal applicator ACTIQ® 1200 micrograms compressed lozenge with integral oromucosal applicator
(fentanyl citrate)
Your medicine is called one of the above, however it will be known as Actiq throughout this leaflet. This product is available in other strengths.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Actiq is and what it is used for
2. What you need to know before you use Actiq
3. How to use Actiq
4. Possible side effects
5. How to store Actiq
6. Contents of the pack and other information
1. WHAT ACTIQ IS AND WHAT IT IS USED FOR
Actiq contains the active substance fentanyl which is a strong pain-relieving medicine known as an opioid.
The Actiq unit comes as a lozenge on a stick.
• It is used to treat breakthrough pain in adult patients with cancer who are already taking other opioid pain medicines for their persistent (around-the-clock) cancer pain. Breakthrough pain is additional sudden pain that occurs suddenly in spite of your having taken your usual opioid pain-relieving medicines.
• Do not use Actiq if you have not been having a prescribed opioid medicine for persistent (around-the-clock) pain regularly every day, for at least a week. This is because if you are not having such a medicine, using Actiq may increase the chances of your breathing becoming dangerously slow or shallow, or even stopping.
• Do not use Actiq to treat pain from injuries, surgery, headaches or migraines.
2. WHAT YOU NEED TO KNOW BEFORE YOU USE ACTIQ
Do not use Actiq:
• if you are not regularly using a prescribed opioid medicine (e.g. codeine, fentanyl, hydromorphone, morphine, oxycodone, pethidine), every day on a regular schedule, for at least a week, to control your persistent pain. If you have not been using these medicines you must not use Actiq, because it may increase the risk that breathing could become dangerously slow and/or shallow, or even stop.
• if you are allergic to fentanyl or any of the other ingredients of this medicine (listed in Section 6).
• if you are currently taking monoamineoxidase inhibitor (MAOI) medicines for severe depression (or have taken them in the past 2 weeks).
• if you have severe breathing problems or severe lung problems where you have an obstruction.
• if you suffer from short term pain other than breakthrough pain.
Do not use Actiq if any of the above apply to you. If you are not sure, talk to your doctor or pharmacist before using Actiq.
Warning and precautions
Keep using the opioid pain medicine you take for your persistent (around-the-clock) cancer pain during your Actiq treatment.
Talk to your doctor or pharmacist before using Actiq if:
• Your other opioid pain medicine for your persistent (around-the-clock) cancer pain is not stabilised yet.
• You have any illness that affects your breathing (such as asthma, wheezing, or shortness of breath).
• You have a head injury or have had any loss of consciousness.
• You have problems with your heart especially slow heart rate.
• You have liver or kidney problems - this will affect how your system breaks down the medicine.
• You have low blood pressure due to a low amount of fluid in your circulation.
• You have diabetes.
• You are over 65 years old - you may need a lower dose and any dose increase will be reviewed very carefully by your doctor.
• You take antidepressants or antipsychotics, please refer to section using other medicines.
Actiq contains approximately 2 grams of sugar and a frequent consumption exposes you to an increase risk of dental decay that may be serious. Thus, it is important to continue to take good care of your mouth and teeth during treatment with Actiq. If you present such serious local effects consult your doctor.
Children and adolescents
Actiq is not recommended for children below 16 years of age.
Other medicines and Actiq
Do not use this medicine and tell your doctor or pharmacist if you are taking:
• Other fentanyl treatments that have been prescribed for your breakthrough pain in the past. If you still have some of these fentanyl treatments at home, check with your pharmacist how to dispose of them.
Tell your doctor or pharmacist before using Actiq if you are taking or have recently taken or might take any other medicines. This includes medicines obtained without a prescription, including herbal medicines. In particular, tell your doctor or pharmacist if you are taking any of the following medicines:
• Any medicines which might make you sleepy -such as sleeping pills, medicines to treat anxiety, some medicines for allergic reaction (antihistamines), or tranquillisers.
• Some muscle relaxants - such as baclofen, diazepam.
• Any medicines that might affect how your body breaks down Actiq - such as ritonavir or other medicines that help control HIV infection, other so-called 'CYP3A4 inhibitors' such as ketoconazole, itraconazole, or fluconazole (used for fungal infections) and troleandomycin, clarithromycin, or erythromycin (medicines for bacterial infections) and so-called 'CYP3A4 inducers' such as rifampin or rifabutin (medicines for bacterial infections), carbamazepine, phenobarbital or phenytoin (medicines used to treat convulsions/fits).
• Certain types of strong pain killers, called partial agonist/antagonists e.g. buprenorphine, nalbuphine and pentazocine (medicines for treatment of pain). You could experience symptoms of withdrawal syndrome (nausea, vomiting, diarrhoea, anxiety, chills, tremor, and sweating) while using these medicines. If you are due to have surgery requiring a general anaesthetic.
The risk of side effects increases if you are taking medicines such as certain antidepressants or antipsychotics. Actiq may interact with these medicines and you may experience mental status changes (e.g. agitation, hallucinations, coma), and other effects such as body temperature above 38°C, increase in heart rate, unstable blood pressure, and exaggeration of reflexes, muscular rigidity, lack of coordination and/ or gastrointestinal symptoms (e.g nausea, vomiting, diarrhoea). Your doctor will tell you whether Actiq is suitable for you.
Actiq with food, drink and alcohol
• Actiq may be used before or after meals. However do not use during meals.
• You may drink some water before using Actiq to help moisten your mouth. However, do not drink or eat anything while using Actiq.
• Do not drink grapefruit juice while taking Actiq. This is because it may affect the way your body breaks down Actiq.
• Do not drink alcohol while using Actiq. It can increase the chances of getting dangerous side effects.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
You should not use Actiq during child-birth because fentanyl may cause breathing difficulties in the newborn child. There is also a risk of the new-born child having withdrawal symptoms of the medicine if Actiq is used for a long time during pregnancy.
Fentanyl can get into breast milk and may cause side effects in the breast-fed infant. Do not use Actiq if you are breast-feeding. You should not start breast-feeding until at least 5 days after the last dose of Actiq.
Ask your doctor or pharmacist for advice before taking any medicine, if you are pregnant or breastfeeding.
Driving and using machines
This medicine may affect you being able to drive or use any tools or machines. Talk to your doctor about whether it is safe for you to drive, or use any tools or machines in the first few hours after taking Actiq.
Do not drive or use any tools or machines if you: feel sleepy or dizzy; have blurred or double vision; have difficulty in concentrating.
It is important you know how you react to Actiq before driving or using any tools or machines.
The medicine can affect your ability to drive as it may make you sleepy or dizzy.
• Do not drive while taking this medicine until you know how it affects you.
• It is an offence to drive if this medicine affects your ability to drive.
However, you would not be committing an offence if:
• The medicine has been prescribed to treat a medical or dental problem and
• You have taken it according to the instructions given by the prescriber or in the information provided with the medicine and
• It was not affecting your ability to drive safely
Talk to your doctor or pharmacist if you are not sure whether it is safe for you to drive while taking this medicine.
Actiq contains glucose and sucrose (types of sugar)
• If you have been told by your doctor that you cannot tolerate or digest some sugars, talk to your doctor before using Actiq.
• Each lozenge contains about 2 grams of glucose. If you have diabetes, you need to take this into account.
• The glucose in the lozenge may be harmful to the teeth. Always make sure you clean your teeth regularly.
3. HOW TO USE ACTIQ
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
When you first start using Actiq, your doctor will work with you to find the dose that will relieve your breakthrough pain. It is very important that you use Actiq exactly as the doctor tells you.
• Do not change doses of Actiq or your other pain medicines on your own. Change in dose must be prescribed and checked by your doctor.
• If you are not sure about the right dose or if you have questions about taking this medicine, talk to your doctor.
How the medicine gets into your body
When you place the lozenge in your mouth:
• The lozenge dissolves and the active substance is released. It takes around 15 minutes for this to happen.
• The active substance is absorbed through the lining of your mouth, into the blood system.
Taking the medicine like this allows it to be absorbed quickly. This means that it relieves your breakthrough pain quickly.
While the right dose is being found
You should start to feel some relief quickly while you are taking Actiq. However, while you and the doctor are finding out the dose that controls your breakthrough pain, you may not get enough pain relief 30 minutes after starting to use one Actiq unit (15 minutes from when you finish using the Actiq unit). If this happens, your doctor may allow you to use a second Actiq unit of the same strength for that same episode of breakthrough pain. Do not use a second unit unless your doctor tells you to. Never use more than two units to treat a single episode of breakthrough pain.
While the right dose is being found, you may need to have more than one strength of Actiq units at home. However, keep only the strengths of Actiq units you need in the house. This is to stop possible confusion or overdose. Talk to your pharmacist about how to dispose of any Actiq units you do not need.
How many units to use
Once the right dose has been found with your doctor, use 1 unit for an episode of breakthrough pain.
Talk to your doctor if your right dose of Actiq does not relieve your breakthrough pain for several episodes of breakthrough pain in a row. Your doctor will decide if your dose needs to be changed.
Please turn over
Herts WD6 1EE. PL 20492/0468
PL 20492/0469
PL 20492/0470
PL 20492/0471
PL 20492/0472

POM
You must tell your doctor straight away if you are using Actiq more than four times per day. This is because he may wish to change your medicine for your persistent (around-the-clock) pain. When he has done this, when your persistent pain has been controlled, he may need to change your dose of Actiq. For the most effective relief, tell your doctor about your pain and how Actiq is working for you. This is so that the dose can be changed if needed.
How to use the medicine
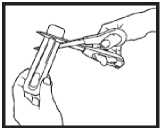
Opening the pack - each Actiq unit is sealed in its own blister pack.
• Open the pack when you are ready to use it. Do not open it in advance.
• Hold the blister pack with the printed side away from you.
• Hold the short tab end of the blister pack.
• Put scissors close to the end of Actiq unit and cut the long tab end completely off (as shown).
• Separate the printed backing from the blister pack and pull the printed backing completely off the blister pack.
• Remove the Actiq unit from the blister pack and put the lozenge in your mouth straight away.
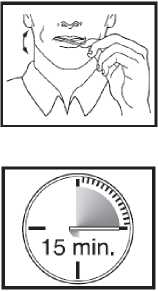
Using the Actiq unit
• Put the lozenge between your cheek and gum.
• Using the handle, keep moving the lozenge round in your mouth, especially along your cheeks. Twirl the handle often.
• To get the most effective relief, finish the lozenge completely in 15 minutes. If you finish too quickly, you will swallow more of the medicine and get less relief from your breakthrough pain.
• Do not bite, suck or chew the lozenge. This would mean lower blood levels and less pain relief than when used as directed.
• If for some reason you are not finishing the whole lozenge each time you have breakthrough pain, talk to your doctor.
How often you should use Actiq
Once a dose is found that effectively controls your pain, do not use more than four Actiq units each day. If you think you might need to use more than four Actiq units per day, talk to your doctor straight away.
How many Actiq units you should use
Do not use more than two units to treat any single episode of breakthrough pain.
If you use more Actiq than you should
The most common side effects of using too much are feeling sleepy, sick or dizzy.
• If you begin to feel dizzy, sick, or very sleepy before the lozenge is completely dissolved, take it out of your mouth and call another person in your house to help you.
A serious side effect of Actiq is slow and/or shallow breathing. This can occur if your dose of Actiq is too high or if you take too much Actiq.
• If this happens, get medical help straight away.
What to do if a child or adult accidentally takes Actiq
If you think someone has accidentally taken Actiq, get medical help straight away. Try to keep the person awake (by calling their name and shaking their arm or shoulder) until emergency help arrives.
If you forget to use Actiq
If you still have the breakthrough pain, you may use Actiq as your doctor has told you. If the breakthrough pain has stopped, do not use Actiq until the next breakthrough pain episode.
If you stop using Actiq
You should discontinue Actiq when you no longer have any breakthrough pain. You must however continue to take your usual opioid pain relieving medicine to treat your persistent cancer pain as advised by your doctor. You may experience withdrawal symptoms similar to the possible side effects of Actiq when discontinuing Actiq. If you experience withdrawal symptoms or if you are concerned about your pain relief you should contact your doctor. Your doctor will evaluate if you need medicine to reduce or eliminate the withdrawal symptoms.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can have side effects, although not everybody gets them. If you notice any of these, contact your doctor. The most serious side effects are shallow breathing, low blood pressure and shock.
You or your carer should REMOVE the Actiq unit from your mouth, contact your doctor immediately and call for emergency help if you experience any of the following serious side effects - you may need urgent medical attention:
• Becoming very sleepy or having slow and/or shallow breathing.
• Difficulty in breathing or dizziness, swelling of the tongue, lip or throat which may be early signs of serious allergic reaction.
Note to Carers:
If you see that the patient taking Actiq has slow and/or shallow breathing or if you have a hard time waking the person up, take the following steps IMMEDIATELY:
• Using the handle, remove the Actiq unit from the person's mouth and keep it out of the reach of children or pets until it is disposed of.
• CALL FOR EMERGENCY HELP.
• While waiting for emergency help, if the person seems to be breathing slowly, prompt them to breathe every 5-10 seconds.
If you feel excessively dizzy, sleepy or otherwise ill while using Actiq, use the handle to remove the lozenge and dispose of it according to the instructions given in this leaflet (see Section 5).
Then contact your doctor for further directions on using Actiq.
Very common side effects (affecting more than 1 in 10 people)
• Vomiting, nausea/feeling sick, constipation, stomach (abdominal) pain
• Asthenia (weakness), sleepiness, dizziness, headaches
• Shortness of breath
Common side effects (affecting 1 to 10 people in 100)
• Confusion, anxiety, seeing or hearing things that are not there (hallucinations), depression, mood swings
• Feeling unwell
• Muscle jerks, feeling of dizziness or "spinning", loss of consciousness, sedation, tingling or numbness, difficulty coordinating movements, increased or altered sensitivity to touch, convulsions (fits)
• Dry mouth, mouth inflammation, tongue problems (for example, burning sensation or ulcers), taste alteration
• Wind, abdominal bloating, indigestion, decreased appetite, weight loss
• Blurred or double vision
• Sweating, skin rash, itchy skin
• Difficulty passing urine
• Accidental injury (for example, falls)
Uncommon side effects (affecting 1 to 10 people in 1,000)
• Tooth decay, paralysis of the gut, mouth ulcers, gum bleeding
• Coma, slurred speech
• Abnormal dreams, feeling detached, abnormal thinking, excessive feeling of well being
• Widening of blood vessels
• Hives
Frequency not known
The following side effects have also been reported with the use of Actiq lozenge but it is not known how often they may occur:
• Receding gums, inflammation of the gum, tooth loss, severe breathing problems, flushing, feeling very warm, diarrhoea, swelling of arms or legs, fatigue, insomnia, pyrexia, withdrawal syndrome (may manifest by the occurrence of the following side effects nausea, vomiting, diarrhoea, anxiety, chills, tremor, and sweating).
Whilst using Actiq you may experience irritation, pain and ulcer at the application site and gum bleeding.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE ACTIQ
The pain-relieving medicine in Actiq is very strong and could be life-threatening if taken accidentally by a child. Actiq must be kept out of the sight and reach of children.
• Do not use Actiq after the expiry date shown on the package label and the carton.
• Do not store above 30°C.
• Store in protective blister until ready for use. Do not use if the blister package has been damaged or opened before you are ready to use it.
• If you are no longer using Actiq, or if you have unused Actiq units in your home, return all unused packs to your doctor or pharmacist.
How to dispose of Actiq after use
Partially used Actiq lozenge may contain enough medicine to be harmful or life-threatening to a child. Even if there is a little or no medicine left on the handle, the handle itself must be properly disposed of as follows:
• If the medicine is totally gone, throw the handle away in a waste container that is out of reach of children and pets.
• If any medicine remains on the handle, place the lozenge under hot running water to dissolve the remainder and then throw the handle away in a waste container that is out of the reach of children and pets.
• If you do not finish the entire lozenge and you cannot immediately dissolve the remaining medicine, put the lozenge out of the reach of children and pets until such a time as you can dispose of the partially used lozenge as instructed above.
• Do not flush partially used lozenge, handles, or the blister packaging down the toilet.
6. FURTHER INFORMATION
What Actiq contains:
Each Actiq 200 micrograms compressed lozenge with integral oromucosal applicator contains 200 micrograms fentanyl (as citrate).
Each Actiq 400 micrograms compressed lozenge with integral oromucosal applicator contains 400 micrograms fentanyl (as citrate).
Each Actiq 600 micrograms compressed lozenge with integral oromucosal applicator contains 600 micrograms fentanyl (as citrate).
Each Actiq 800 micrograms compressed lozenge with integral oromucosal applicator contains 800 micrograms fentanyl (as citrate).
Each Actiq 1200 micrograms compressed lozenge with integral oromucosal applicator contains 1200 micrograms fentanyl (as citrate).
The other ingredients are:
Lozenge:
Dextrates hydrated (equivalent to approximately 2 grams of glucose). Citric acid, disodium phosphate, artificial berry flavour (maltodextrin, propylene glycol, artificial flavours, and triethylcitrate), magnesium stearate.
Edible glue used to attach the lozenge to the handle Modified maize based food starch, confectioner's sugar (as sucrose and maize starch), water. Imprinting ink
Water, de-waxed white shellac, propylene glycol, blue synthetic coal tar dye (E133)
What Actiq looks like and contents of the pack
Actiq 200 micrograms compressed lozenge with integral oromucosal applicator are white to off-white solid lozenge attached to a handle for oromucosal application. The lozenge is marked with 'ACTIQ 200' in blue and the handle is marked with a dark grey band and 'ACTIQ® 200pg' in black print.
Actiq 400 micrograms compressed lozenge with integral oromucosal applicator are white to off-white solid lozenge attached to a handle for oromucosal application. The lozenge is marked with 'ACTIQ 400' in blue and the handle is marked with a blue band and 'ACTIQ® 400pg' in black print.
Actiq 600 micrograms compressed lozenge with integral oromucosal applicator are white to off-white solid lozenge attached to a handle for oromucosal application. The lozenge is marked with 'ACTIQ 600' in blue and the handle is marked with a orange band and 'ACTIQ® 600pg' in black print.
Actiq 800 micrograms compressed lozenge with integral oromucosal applicator are white to off-white solid lozenge attached to a handle for oromucosal application. The lozenge is marked with 'ACTIQ 800' in blue and the handle is marked with a purple band and 'ACTIQ® 800pg' in black print.
Actiq 1200 micrograms compressed lozenge with integral oromucosal applicator are white to off-white solid lozenge attached to a handle for oromucosal application. The lozenge is marked with 'ACTIQ 1200' in blue and the handle is marked with a green band and 'ACTIQ® 1200pg' in black print.
The lozenge may appear slightly mottled on storage. This is due to slight changes in the flavouring agent of the product and does not affect how the product works in any way. However, if you notice any other signs of deterioration, please refer to your doctor or pharmacist.
Actiq is supplied in individual blister packages. Blister packages are supplied in cartons of 3 or 30 individual Actiq units.
Actiq is manufactured by Cephalon France, 5 rue Charles Martigny, 94700 Maisons-Alfort, France or Teva Pharmaceuticals B.V Europe, Swensweg 5, 2031 GA Haarlem, The Netherlands and is procured from within the EU and repackaged in the UK by the Product Licence holder: CD Pharma Ltd, Unit 3, Manor Point, Manor Way, Borehamwood,
Actiq 200 micrograms compressed lozenge with integral oromucosal applicator Actiq 400 micrograms compressed lozenge with integral oromucosal applicator Actiq 600 micrograms compressed lozenge with integral oromucosal applicator Actiq 800 micrograms compressed lozenge with integral oromucosal applicator Actiq 1200 micrograms compressed lozenge with integral oromucosal applicator
Date of preparation: 1st July 2016
Actiq is a registered trademark of Cephalon, Inc. or one of its affiliates.