Acular
Out of date information, search anotherReporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at:www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Acular
Keep out of the sight and reach of children
Do not use the product if the tamper proof seal on the bottle is broken.
Do not use the product after the expiry date. (This is printed on both the label on the bottle and on the bottom of the carton). The expiry date refers to the last day of that month.
Throw the bottle away 28 days after opening, even if there is solution remaining.
If your doctor tells you to stop using this medication, take any unused solution back to your pharmacist for safe disposal. Only keep it if your doctor tells you to.
If the solution becomes discoloured or shows any signs of deterioration, seek the advice of your pharmacist.
6. Contents of the pack and other information What Acular contains
• Each ml of eye drops solution contains 0.5% of ketorolac trometamol as the active ingredient.
• It also contains: benzalkonium chloride, disodium edetate, octoxynol 40, sodium chloride, sodium hydroxide or hydrochloric acid (to adjust pH) and purified water.
What Acular looks like and contents of the pack
Acular is a clear, colourless to slightly yellow solution and is contained in a bottle with a dropper applicator.
Each bottle contains 5ml of the medicine.
Manufactured by: Allergan SA., Av. De la Industria 24, Tres Cantos, 1300-278, Madrid, Spain.
Procured from within the EU and repackaged by the Product Licence holder:
B&S Healthcare, Unit 4, Bradfield Road, Ruislip, Middlesex, HA4 ONU, UK.
POM
Acular® 0.5% w/v Ophthalmic Solution PL No: 18799/1713
Leaflet date: 06.11.2014
Acular is a registered trademark of Allergan, Inc.
Acular® 0.5% w/v Ophthalmic Solution
(ketorolac trometamol)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
The name of your medicine is Acular 0.5% w/v Ophthalmic Solution but will be referred to as Acular throughout this leaflet.
What is in this leaflet:
1. What Acular is and what it is used for
2. What you need to know before you use Acular
3. How to use Acular
4. Possible side effects
5. How to store Acular
6. Contents of the pack and other information
1. What Acular is and what it is used for
Acular is used to prevent and relieve eye inflammation following surgery on the eye in adults.
Acular belongs to a group of medicines known as non-steroidal anti-inflammatory drugs (NSAIDs). The active ingredient in Acular is ketorolac trometamol.
2. What you need to know before you use Acular Do not use Acular
• If you are allergic to ketorolac, or any of the other ingredients of this medicine (listed in section 6).
• If you are allergic to aspirin or any other similar drugs.
Warnings and precautions
If any of the following apply talk to your doctor before using Acular.
If you suffer from, or have in the past suffered from:
• viral or bacterial infections of the eye
• bleeding tendencies (for example, anaemia) or stomach ulcers
• diabetes
• rheumatoid arthritis
• dry eye syndrome
• asthma after using non-steroidal anti-inflammatories
• or if you have had recent eye surgery.
Other medicines and Acular
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicine, including medicines obtained without a prescription.
If you use Acular with another eye medicine, leave at least 5 minutes between putting in Acular and the other medicine.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before using this medicine. Acular should not be used if you are pregnant or are breast-feeding, unless your doctor recommends it.
Children
Acular should not be prescribed for use in children.
Driving and using machines
Acular may cause temporary blurred vision. Do not drive or use machinery until the symptoms have cleared.
Acular contains benzalkonium chloride
• If you wear contact lenses you should remove them prior to application and wait at least 15 minutes before reinsertion.
• The preservative in Acular benzalkonium chloride, may cause eye irritation and can permanently damage this type of lens. Acular is known to discolour soft contact lenses.
• Avoid contact with soft contact lenses.
3. How to use Acular
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure. The recommended dose is 1 drop into the affected eye(s), 3 times a day, starting 24 hours before surgery and continuing for up to 3 weeks after eye surgery.
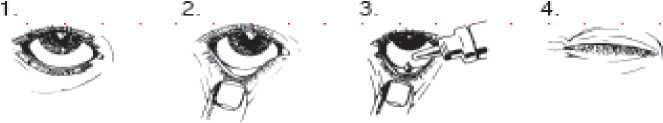
Instructions for use
• You must not use the bottle if the tamper-proof seal on the bottle neck is broken before you first use it. Apply your eye drops in the following way:
1. Wash your hands. Tilt your head back and look at the ceiling.
2. Gently pull the lower eyelid down until there is a small pocket.
3. Turn the bottle upside down and squeeze it to release one drop into each eye that needs treatment.
4. Let go of the lower lid, and close your eye for 30 seconds.
If a drop misses your eye, try again.
To avoid contamination or injury, do not let the tip of the dropper touch your eye or anything else.
Replace and tighten the cap straight after use.
Wipe off any excess liquid from your cheek with a clean tissue.
The proper application of your eye drops is very important. If you have any questions ask your doctor or pharmacist.
If you use more Acular than you should
The application of too many drops is unlikely to lead to unwanted side effects.
Apply your next dose at the normal time. If, by accident, anyone drinks this medicine, drink fluids to dilute and contact your doctor.
If you forget to use Acular
If you forget a dose apply it as soon as you remember, unless it is almost time for your next dose, in which case you should miss out the forgotten dose. Then take your next dose as usual and continue with your normal routine.
Do not take a double dose to make up for a forgotten dose.
If you stop using Acular
Acular should be used as advised by your doctor. If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects related to the cornea (the surface of the eye) may be more likely if Acular is used for longer than two weeks or if you are using topical steroid drops at the same time or if you have a related eye condition. You should see your doctor immediately if you experience pain, increased irritation in the eye or changes in vision.
The very common side effects (occurring in more than 1 in 10 patients) are:
Irritation of the eye, stinging and/or burning in the eye, eye pain.
Common side effects (occurring in between 1 and 10 patients in every 100) are: allergic reaction, eye and/or eyelid swelling/puffiness, itchy eyes, red eye, infection of the eye, inflammation of the eye (surface or inside), bleeding of the retina, swelling of central retina (light-sensitive layer of the eye), headache, accidental injury caused by the tip of the dropper touching the eye, increased pressure in the eye, blurred and/or diminished vision.
Uncommon side effects (occurring in between 1 and 10 patients in every 1,000) are: inflammation or damage to the front clear layer of the eye, eye dryness and/or watery eyes.
Not known side effects (cannot be estimated from the available data) are: damage on the surface of the eye such as thinning, erosion, degradation of cell(s), difficulty in breathing or wheezing, aggravation of asthma.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at:www.mhra.gov.uk/yellowcard. By reporting side effects, you can help provide more information on the safety of this medicine
5. How to store Ketorolac
Keep out of the sight and reach of children
Do not use the product if the tamper proof seal on the bottle is broken.
Do not use the product after the expiry date. (This is printed on both the label on the bottle and on the bottom of the carton). The expiry date refers to the last day of that month.
Throw the bottle away 28 days after opening, even if there is solution remaining.
If your doctor tells you to stop using this medication, take any unused solution back to your pharmacist for safe disposal. Only keep it if your doctor tells you to.
If the solution becomes discoloured or shows any signs of deterioration, seek the advice of your pharmacist.
6. Contents of the pack and other information What Ketorolac contains
• Each ml of eye drops solution contains 0.5% of ketorolac trometamol as the active ingredient.
• It also contains: benzalkonium chloride, disodium edetate, octoxynol 40, sodium chloride, sodium hydroxide or hydrochloric acid (to adjust pH) and purified water.
What Ketorolac looks like and contents of the pack
Ketorolac is a clear, colourless to slightly yellow solution and is contained in a bottle with a dropper applicator.
Each bottle contains 5ml of the medicine.
Manufactured by: Allergan SA., Av. De la Industria 24, Tres Cantos, 1300-278, Madrid, Spain.
Procured from within the EU and repackaged by the Product Licence holder:
B&S Healthcare, Unit 4, Bradfield Road, Ruislip, Middlesex, HA4 ONU, UK.
POM
Ketorolac 0.5% w/v Ophthalmic Solution PL No: 18799/1713
Leaflet date: 06.11.2014
Ketorolac Trometamol 0.5% w/v Ophthalmic Solution
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
The name of your medicine is Ketorolac Trometamol 0.5% w/v Ophthalmic Solution but will be referred to as Ketorolac throughout this leaflet.
What is in this leaflet:
1. What Ketorolac is and what it is used for
2. What you need to know before you use Ketorolac
3. How to use Ketorolac
4. Possible side effects
5. How to store Ketorolac
6. Contents of the pack and other information
1. What Ketorolac is and what it is used for
Ketorolac is used to prevent and relieve eye inflammation following surgery on the eye in adults.
Ketorolac belongs to a group of medicines known as non-steroidal anti-inflammatory drugs (NSAIDs). The active ingredient in Ketorolac is ketorolac trometamol.
2. What you need to know before you use Ketorolac Do not use Ketorolac
• If you are allergic (hypersensitive) to ketorolac, or any of the other ingredients of this medicine (listed in section 6).
• If you are allergic to aspirin or any other similar drugs.
Warnings and precautions
If any of the following apply talk to your doctor before using Ketorolac.
If you suffer from, or have in the past suffered from,
• viral or bacterial infections of the eye
• bleeding tendencies (for example, anaemia) or stomach ulcers
• diabetes
• rheumatoid arthritis
• dry eye syndrome
• asthma after using non-steroidal anti-inflammatories
• or if you have had recent eye surgery.
Other medicines and Ketorolac
Please tell your doctor or pharmacist if you are using, have recently used or might use any other medicine, including medicines obtained without a prescription.
If you use Ketorolac with another eye medicine, leave at least 5 minutes between putting in Ketorolac and the other medicine.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before using this medicine.
Ketorolac should not be used if you are pregnant or might become pregnant or are breast-feeding, unless your doctor recommends it.
Children
Ketorolac should not be prescribed for use in children.
Driving and using machines
Ketorolac may cause temporary blurred vision. Do not drive or use machinery until the symptoms have cleared.
Important information about some of the ingredients of Ketorolac
• If you wear contact lenses you should remove them prior to application and wait at least 15 minutes before reinsertion.
• The preservative in Ketorolac benzalkonium chloride, may cause eye irritation and can permanently damage this type of lens. Ketorolac is known to discolour soft contact lenses.
• Avoid contact with soft contact lenses.
3. How to use Ketorolac
Always use this medicine exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure. The recommended dose is 1 drop into the affected eye(s), 3 times a day, starting 24 hours before surgery and continuing for up to 3 weeks after eye surgery.
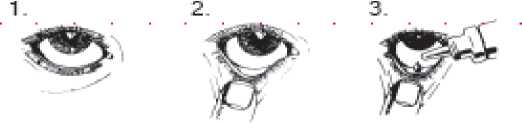
4.
Instructions for use
• You must not use the bottle if the tamper-proof seal on the bottle neck is broken before you first use it. Apply your eye drops in the following way:
1. Wash your hands. Tilt your head back and look at the ceiling
2. Gently pull the lower eyelid down until there is a small pocket
3. Turn the bottle upside down and squeeze it to release one drop into each eye that needs treatment
4. Let go of the lower lid, and close your eye for 30 seconds If a drop misses your eye, try again.
To avoid contamination or injury, do not let the tip of the dropper touch your eye or anything else.
Replace and tighten the cap straight after use.
Wipe off any excess liquid from your cheek with a clean tissue.
The proper application of your eye drops is very important. If you have any questions ask your doctor or pharmacist.
If you use more Ketorolac than you should
The application of too many drops is unlikely to lead to unwanted side effects.
Apply your next dose at the normal time. If, by accident, anyone drinks this medicine, drink fluids to dilute and contact your doctor.
If you forget to use Ketorolac
If you forget a dose apply it as soon as you remember, unless it is almost time for your next dose, in which case you should miss out the forgotten dose. Then take your next dose as usual and continue with your normal routine.
Do not take a double dose to make up for a forgotten dose.
If you stop using Ketorolac
Ketorolac should be used as advised by your doctor. If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects related to the cornea (the surface of the eye) may be more likely if Ketorolac is used for longer than two weeks or if you are using topical steroid drops at the same time or if you have a related eye condition. You should see your doctor immediately if you experience pain, increased irritation in the eye or changes in vision.
The very common side effects (occurring in more than 1 in 10 patients) are:
Irritation of the eye, stinging and/or burning in the eye, eye pain.
Common side effects (occurring in between 1 and 10 patients in every 100) are: allergic reaction, eye and/or eyelid swelling/puffiness, itchy eyes, red eye, infection of the eye, inflammation of the eye (surface or inside), bleeding of the retina, swelling of central retina (light-sensitive layer of the eye), headache, accidental injury caused by the tip of the dropper touching the eye, increased pressure in the eye, blurred and/or diminished vision.
Uncommon side effects (occurring in between 1 and 10 patients in every 1,000) are: inflammation or damage to the front clear layer of the eye, eye dryness and/or watery eyes.
Not known side effects (cannot be estimated from the available data) are: damage on the surface of the eye such as thinning, erosion, degradation of cell(s), difficulty in breathing or wheezing, aggravation of asthma.