Adrenaline 1Mg/10Ml (1:10 000) Solution For Injection In Pre-Filled Syringe
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Adrenaline 1 mg/10 ml (1:10,000), solution for injection in pre-filled syringe
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml of solution for injection contains 0.1 mg of adrenaline (as adrenaline tartrate) Each 10 ml pre-filled syringe contains 1 mg adrenaline (as adrenaline tartrate)
Excipient with known effect: sodium
Each ml of solution for injection contains 3.54 mg equivalent to 0.154 mmol of sodium.
Each 10 ml pre-filled syringe contains 35.4 mg equivalent to 1.54 mmol of sodium. For a full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Solution for injection in pre-filled syringe
Clear and colourless solution in a 10 ml pre-filled syringe
pH = 3.0 to 3.4
Osmolarity: 270 - 300 mOsm/l
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Cardiopulmonary resuscitation
Acute anaphylaxis in adults
4.2 Posology and method of administration
Intravenous adrenaline should only be administered by those experienced in the use and titration of vasopressors in their normal clinical practice.
Cardiopulmonary resuscitation: 10 ml of the 1:10,000 solution (1 mg) by the intravenous or intraosseous route, repeated every 3-5 minutes until return of spontaneous circulation.
Endotracheal use should only be considered as a last resort if no other route of administration is accessible, at a dose of 20 to 25 ml of the 1: 10,000 solution (2 to 2.5 mg).
In cardiac arrest following cardiac surgery, Adrenaline should be administered intravenously in doses of 0.5 ml or 1 ml of 1:10,000 solution (50 or 100 micrograms) very cautiously and titrated to effect.
Acute anaphylaxis
Titrate using intravenous boluses of 0.5 ml 1:10,000 solution (0.05 mg) according to response.
Adrenaline 1 mg/10 ml (1:10,000) solution for injection in pre-filled syringe is not recommended for intramuscular use in acute anaphylaxis. For intramuscular administration, a 1 mg/ml (1:1000) solution should be used
Paediatric Population:
This medicinal product is not suitable for delivering a dose of less than 0.5 ml and should therefore not be used by the intravenous or intraosseous route, in neonates and infants with body weight less than 5 kg.
Cardiac arrest in children:
Intravenous or intraosseous route (above 5 kg only): 0.1 ml/kg of 1:10,000 solution (10 micrograms/kg) to a maximum single dose of 10 ml of 1:10,000 solution (1 mg), repeated every 3-5 minutes until return of spontaneous circulation.
Endotracheal use (any body weight) should only be considered as a last resort if no other route of administration is accessible, at a dose of 1 ml/kg of 1:10,000 solution (100 micrograms/kg) to a maximum of single dose of 25 ml of 1:10,000 solution (2.5 mg).
4.3 Contraindications
Patients with known hypersensitivity to an excipient, where an alternative presentation of adrenaline or alternative vasopressor is available.
4.4 Special warnings and precautions for use
Adrenaline 1 mg/10 ml (1:10,000), solution for injection in pre-filled syringe is indicated for emergency treatment. Medical supervision is necessary after administration.
For intramuscular administration, a 1 mg/ml (1:1000) solution should be used.
In the treatment of anaphylaxis and in other patients with a spontaneous circulation, intravenous adrenaline can cause life-threatening hypertension, tachycardia, arrhythmias and myocardial ischaemia.
Intravenous adrenaline should only be used by those experienced in the use and titration of vasopressors in their normal clinical practice. Patients who are given IV adrenaline require continuous monitoring of ECG, pulse oximetry and frequent blood pressure measurements as a minimum.
The risk of toxicity is increased if the following conditions are pre-existing
• Hyperthyroidism
• Hypertension
• Structural cardiac disease, cardiac arrhythmias, severe obstructive cardiomyopathy,
• Coronary insufficiency
• Phaeochromocytoma,
• Hypokalaemia
• Hypercalcaemia
• Severe renal impairment
• Cerebrovascular disease, organic brain damage or arteriosclerosis
• Patients taking Monoamine oxidase (MAO) inhibitors (see section 4.5)
• Patients taking concomitant medication which results in additive effects, or sensitizes the myocardium to the actions of sympathomimetic agents (see section 4.5)
Prolonged use of adrenaline can result in severe metabolic acidosis because of elevated blood concentrations of lactic acid.
Adrenaline may increase intra-ocular pressure in patients with narrow angle glaucoma.
Adrenaline should be used with caution in patients with prostatic hyperplasia with urinary retention.
Adrenaline may cause or exacerbate hyperglycaemia, blood glucose should be monitored, particularly in diabetic patients.
Adrenaline should be used with caution in elderly patients.
Adrenaline should not be used during the second stage of labour (See Section 4.6).
This medicinal product contains 3.54 mg of sodium per ml of solution for injection: to be taken into consideration by patients on strict sodium diet.
4.5 Interaction with other medicinal products and other forms of interaction Volatile halogen anaesthetics: severe ventricular arrhythmia (increase in cardiac excitability).
Imipramine antidepressants: paroxysmal hypertension with the possibility of arrhythmia (inhibition of the entry of sympathomimetics into sympathetic fibres).
Serotoninergic-adrenergic antidepressants: paroxysmal hypertension with the possibility of arrhythmia (inhibition of the entry of sympathomimetics into sympathetic fibres).
Sympathomimetic agents: concomitant administration of other sympathomimetic agents may increase toxicity due to possible additive effects.
Non-selective MAO inhibitors: increased pressor action of adrenaline, usually moderate.
Selective MAO-A inhibitors, Linezolid (by extrapolation from non-selective MAO inhibitors): Risk of aggravation of pressor action.
Alpha-adrenergic blocking agents: Alpha-blockers antagonise the vasoconstriction and hypertension effects of adrenaline, increasing the risk of hypotension and tachycardia.
Beta-adrenergic blocking agents: Severe hypertension and reflex bradycardia may occur with non-cardioselective beta-blocking agents. Beta-blockers, especially non-cardioselective agents, also antagonise the cardiac and bronchodilator effects of adrenaline.
Insulin or oral hypoglycaemic agents: Adrenaline-induced hyperglycaemia may lead to loss of blood-sugar control in diabetic patients treated with insulin or oral hypoglycaemic agents.
4.6 Fertility, pregnancy and lactation
Pregnancy:
Teratogenic effect has been demonstrated in animal experiments.
Adrenaline should only be used during pregnancy if the potential benefits outweigh the possible risks to the foetus. If used during pregnancy, adrenaline may cause anoxia to the foetus.
Adrenaline usually inhibits spontaneous or oxytocin induced contractions of the uterus and may delay the second stage of labour. In dosage sufficient to reduce uterine contractions, adrenaline may cause a prolonged period of uterine atony with haemorrhage. For this reason parenteral adrenaline should not be used during the second stage of labour.
Lactation:
Adrenaline is distributed into breast milk. Breast-feeding should be avoided by mothers receiving adrenaline.
Fertility
No information available concerning impact of adrenaline on fertility.
4.7 Effects on ability to drive and use machines
Not applicable in normal conditions of use.
4.8 Undesirable effects
Metabolism and nutrition disorders:
Frequency not known: hyperglycaemia, hypokalaemia, metabolic acidosis.
Psychiatric disorders:
Frequency not known: anxiety, nervousness, fear, hallucinations.
Nervous system disorders:
Frequency not known: headache, tremors, dizziness, syncope.
Eye disorders:
Frequency not known: mydriasis.
Cardiac disorders:
Frequency not known: palpitations, tachycardia. In high dosage or for patients sensitive to adrenaline: cardiac dysrhythmia (sinus tachycardia, ventricular fibrillation/cardiac arrest), acute angina attacks, and risk of acute myocardial infarction.
Vascular disorders:
Frequency not known: pallor, coldness of the extremities. In high dosage or for patients sensitive to adrenaline: hypertension (with risk of cerebral haemorrhage), vasoconstriction (for example cutaneous, in the extremities or kidneys).
Respiratory, thoracic and mediastinal disorders:
Frequency not known: dyspnoea.
Gastrointestinal disorders:
Frequency not known: nausea, vomiting.
General disorders and administration site conditions:
Frequency not known: sweating, weakness
Repeated local injections may produce necrosis at sites of injection as a result of vascular constriction.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via Yellow Card Scheme.
Website: www.mhra.gov .uk/yellowcard
4.9 Overdose
Over dosage or inadvertent intravenous administration of adrenaline may produce severe hypertension. Cerebral, cardiac or vascular accidents which could be potentially fatal may occur as a result (cerebral haemorrhage, dysrhythmias such as transient bradycardia followed by tachycardia that may result in arrhythmia, myocardial necrosis, acute pulmonary oedema, renal insufficiency).
The effects of adrenaline may be counteracted, depending on the condition of the patient, by administration of quick-acting vasodilators, of quick-acting alpha-adrenoreceptor blocking agents (e.g. phentolamine), or beta-adrenoreceptor blocking agents (e.g. propanolol). However, due to the short half-life of adrenaline, treatment with these medicines may not be necessary. In case of prolonged hypotensive reaction, administration of another vasopressive agent such as noradrenaline may be required.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: adrenergic and dopaminergic agents, adrenaline
ATC code: C01 CA 24
Adrenaline is a direct acting sympathomimetic agent, which exerts effects on both a and P adrenoceptors. It has more pronounced effects on p than on a adrenoceptors, although a effects prevail at high doses.
The effects of adrenaline include increased rate and force of cardiac contraction, cutaneous vasoconstriction and broncho-dilatation. With higher doses, stimulation of peripheral a receptors results in an increase in peripheral resistance and in blood pressure.
5.2 Pharmacokinetic properties
Pharmacologically active concentrations of adrenaline are not achieved following oral administration as it is rapidly oxidised and conjugated in the gastrointestinal mucosa and the liver. Absorption from subcutaneous tissue is slow due to local vasoconstriction; effects are produced within 5 minutes. Absorption is more rapid after intramuscular injection than after subcutaneous injection.
Adrenaline is rapidly distributed into the heart, spleen, several glandular tissues and adrenergic nerves. It readily crosses the placenta and is approximately 50% bound to plasma proteins.
Adrenaline is rapidly inactivated in the body, mostly in the liver by the enzymes catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO). Most of a dose of adrenaline is excreted as metabolites in urine.
After intravenous administration, the plasma half-life is about 2-3 minutes.
5.3 Preclinical safety data
There are no pre-clinical data of relevance to the prescriber, which are additional to that already included in other sections of the SPC.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Sodium chloride
Hydrochloric acid (for pH adjustment)
Sodium hydroxide (for pH adjustment)
Water for injections
6.2 Incompatibilities
In the absence of compatibility studies, this product must not be mixed with other
medicinal products.
6.3
Shelf life
Unopened: 18 months.
6.4 Special precautions for storage
Do not store above 25°C.
Do not freeze.
Store in the aluminium pouch in order to protect from light and oxygen.
6.5 Nature and contents of container
10 ml solution in a polypropylene pre-filled syringe without a needle, individually packaged in a transparent blister and overwrapped in an aluminium pouch containing an oxygen absorbing sachet. Available in a box of 1 or 10.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
The aluminium pouch and syringe blister should only be opened immediately prior administration.
After opening the pouch the product must be used immediately.
The external surface of the syringe and its content are sterile if the blister is unopened and undamaged.
Strictly respect the protocol below
The pre-filled syringe is for single patient use only. Discard the syringe after use. Do not reuse.
The product should be inspected visually for particles and discolouration prior to administration. Only clear colourless solution free from particles or precipitates should be used.
The product should not be used if the pouch or the blister has been opened or if the tamper evident seal on the syringe (plastic film at the basis of the end cap) is broken.
1) Tear open the aluminium pouch by hand only using the indent(s) Do not use sharp instruments to open the pouch.
2) Withdraw the pre-filled syringe from the sterile blister.
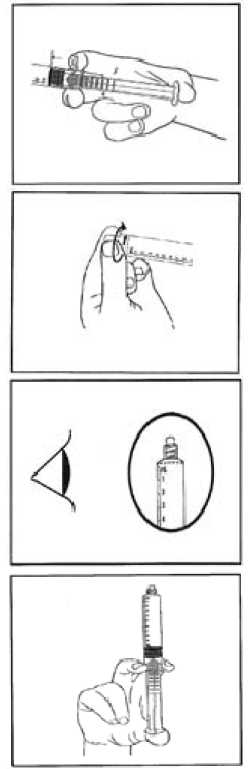
3) Push on the plunger to free the bung.
The sterilisation process may have caused adhesion of the bung to the body of the syringe.
4) Twist off the end cap to break the seals.
Do not touch the exposed luer connection in order to avoid contamination.
5) Check the syringe seal tip has been completely removed.
If not, replace the cap and twist again.
6) Expel the air by gently pushing the plunger.
7) Connect syringe to vascular access device or to needle.
Push the plunger to inject the required volume.
Any unused product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Laboratoire Aguettant 1 rue Alexander Fleming 69007 LYON FRANCE
8 MARKETING AUTHORISATION NUMBER(S)
PL 14434/0031
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10/12/2015
10 DATE OF REVISION OF THE TEXT
22/08/2016