Airomir Inhaler Pressurised Inhalation Suspension
|
TEVA UK Ref: 231-30-87783-E LEA SALBUTAMOL (AIROMIR) 100MCG/D AUTO INH 200 TUK <3M |
Version: 3 |
12 February 2016 |
—Airomir® Autohaler® device Salbutamol
PACKAGE LEAFLET: INFORMATION FOR THE USER

Read all of this leaflet carefully before you start taking this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Airomir Autohaler device is and what it is used for
2. What you need to know before you use Airomir Autohaler device
3. How to use Airomir Autohaler device
4. Possible side effects
5. How to store Airomir Autohaler device
6. Contents of the pack and other information
1. What Airomir Autohaler device is and what it is used for
Airomir Autohaler device contains salbutamol, which is a bronchodilator. It opens up the breathing tubes of the lungs and makes breathing easier. Salbutamol can help you feel less tight-chested and less breathless or wheezy.
Airomir Autohaler device is used:
• to treat asthma by relieving wheezing and shortness of breath
• to prevent asthma following exercise
• for the treatment of wheezing and shortness of breath caused by certain other chest diseases.
2. What you need to know before you use Airomir Autohaler device
Do not use Airomir Autohaler device and consult your doctor if you:
• are allergic (hypersensitive) to salbutamol or any of the other ingredients of this medicine.
Airomir Autohaler device should NOT be used to treat premature labour or threatened miscarriage, as inhaled salbutamol products are not suitable for this purpose.
Warnings and precautions
Talk to your doctor or pharmacist or nurse before using this medicine if you:
• have thyroid problems
• have diabetes
• have serious heart disease
• suffer from fast irregular heart rhythms or high blood pressure
• have acute severe asthma
• have hypoxia (lack of oxygen in the body)
• have a history of heart disease or angina.
Children and adolescents
Consult a doctor immediately if your usual treatment is not working or if you need more than 8 puffs per day (adult) or 4 puffs per day (children) or in case of worsening asthma symptoms. Your dose or frequency should only be increased on medical advice.
Other medicines and Airomir Autohaler device
Tell your doctor or pharmacist if you are taking any other medicines:
• xanthines e.g. aminophylline or theophylline
• metronidazole
• disulfiram
• steroids
• water tablets (diuretics)
• long-term laxatives
• beta-blockers e.g. propranolol
• medicines used to treat heart disease e.g. digoxin
• monoamine oxidase-inhibitors (anti-depressants) e.g. phenelzine
• tricyclic antidepressants e.g. amitriptyline or trazodone.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including any medicines obtained without a
prescription.
If you are having treatment that requires a general anaesthetic, please tell your anaesthetist that you are taking Airomir Autohaler device. Do not use this medicine for at least six hours before the intended anaesthetic.
Airomir Autohaler device contains ethanol
• Airomir Autohaler device contains a small amount of ethanol (alcohol).
Pregnancy and breast-feeding
• If you are pregnant, planning to become pregnant or are breast-feeding, ask your doctor for advice before taking this medicine. Airomir Autohaler device should only be used if recommended by a doctor.
Driving and using machines
• Airomir Autohaler device may cause dizziness. If you are affected, DO NOT drive or operate machinery.
3.How to use Airomir Autohaler device
Always use Airomir Autohaler device exactly as your doctor or pharmacist has told you. You should check with your doctor or pharmacist if you are not sure.
If you see any other doctor or dentist, please tell them that you are using Airomir Autohaler device.
Use in children and adolescents
The recommended dose is:
Adults (including the elderly)
• For the relief of symptoms such as wheezing and shortness of breath
Either one or two puffs as needed.
• For asthma due to exercise
Two puffs before exercising.
Do not take more than eight puffs in 24 hours.
Children (aged 4 to 11 years)
• For the relief of symptoms such as wheezing and shortness of breath
Usually one puff as needed. This may be increased to two puffs if necessary.
• For asthma due to exercise
Usually one puff before exercising. This may be increased to two puffs if necessary.
• For chronic therapy
Usually up to two puffs four times daily.
Children (aged 12 years and over)
Dose as per adult population.
Children using this inhaler should be supervised by an adult and should use this inhaler only as advised by the doctor.
All patients
• If you have taken a dose of two puffs, you should wait four hours before taking another dose. DO NOT take more than eight puffs in 24 hours.
• If you are not sure how many puffs to take or how often to take them, please ask your doctor or pharmacist.
If your usual treatment is not working or you need more than eight puffs per day, please tell your doctor. You should NOT increase your dose without consulting your doctor.
If you use more of your Airomir Autohaler device than you should
If you accidentally take more puffs than your doctor told you to, please contact your nearest hospital casualty department, or tell your doctor, immediately. You may notice that your heart is beating faster than usual, that you feel shaky or tense, you may have a headache or your skin may look flushed and feel hot. These effects normally wear off in a few hours. Your doctor may want to check your blood potassium levels.
If you stop using Airomir Autohaler device
Do not stop taking your medicine without talking to your doctor first even if you feel better.

Before use:
This inhaler has a different feel and taste to CFC inhalers you may have used before.
If this is a new device or if you have not used it for 2 weeks or more, it must be tested before use by releasing 4 puffs into the air in the following way:
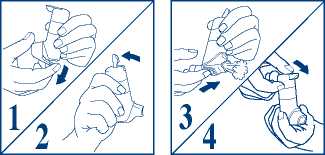
1. Take the cover off from the mouthpiece, by pulling down on the lip at the back (fig. 1).
2. Shake the inhaler vigorously. Point the mouthpiece away from you so that the puffs of medicine will go into the air. Hold the inhaler upright and push the lever up so that it stays up (fig. 2).
3. Then, to release a puff, push the dose release slide on the bottom of the device in the direction indicated by the arrow on it (fig. 3).
4. To release the next puff you need to return the lever to its down position (fig. 4) and follow steps 2 and 3 again. Steps 2 and 3 should be repeated until a total of 4 puffs have been released. After releasing the fourth puff, return the lever to its down position, to be ready to take a puff of your medicine.
REG0068388
Version 5.4
Approved
,ved
Page 1 of 3
DO NOT USE THE DOSE RELEASE SLIDE TO TAKE YOUR MEDICINE, THE AUTOHALER DEVICE WILL AUTOMATICALLY RELEASE A DOSE WHEN YOU BEGIN TO BREATHE IN FROM THE MOUTHPIECE
The instructions for taking a puff are given over the page.
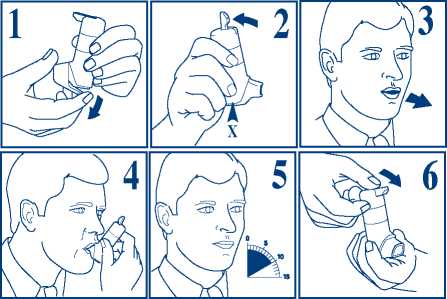
1. Take the cover off from the mouthpiece, by pulling down on the lip at the back.
2. Shake the inhaler vigorously. Hold your Autohaler device upright as shown. Push the lever up so that it stays up. Continue to hold your Autohaler device upright, making sure that your hand is not blocking the air vent (marked by X in fig. 2) at the bottom of the device.
3. Breathe out as far as is comfortable and then immediately place the mouthpiece in your mouth and close your lips around it.
4. Breathe in slowly and deeply through the mouthpiece. Do not stop breathing in when you hear the slight click and feel the puff in your mouth as it is important that you carry on breathing in after the puff is released.
5. Hold your breath for 10 seconds and then breathe out slowly.
6. The lever must be lowered to the down position immediately after each puff whilst holding the Autohaler device upright. If your doctor has prescribed more than one puff, repeat steps 2-6. After use, replace the cover on the mouthpiece.

Cleaning instructions
For normal hygiene, the mouthpiece of your Autohaler device should be cleaned weekly with a clean, dry tissue or cloth.
CAUTION: Do not push a drying cloth or anything else into any part of the device since it may cause damage to its operating parts. Do not take the device apart.
How to tell when your device is empty
When the device is completely empty you will not feel or hear any propellant being discharged.
Further questions
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Airomir Autohaler device can cause side effects, although not everybody gets them.
If, immediately after inhaling a puff, your asthma worsens, do not use Airomir Autohaler device again and contact your doctor straight away.
If the following happens, stop using Airomir Autohaler device and tell your doctor immediately or go to the casualty department at your nearest hospital:
• an allergic reaction (swelling of the lips, face or neck leading to severe difficulty in breathing; skin rash or hives, and a fall in blood pressure. You might collapse in very rare circumstances).
This is a very serious but rare side effect. You may need urgent medical attention or hospitalisation.
The following side effects have been reported:
Common: may affect up to 1 in 10 people
• Feeling tense
• Headache
• Dizziness
• Mild tremor (shaking) especially of the hands.
Uncommon: may affect up to 1 in 100 people
• Muscle pain.
Rare: may affect up to 1 in 1,000 people
• Low blood levels of potassium which can cause muscle weakness, twitching or abnormal heart rhythm
• Sleep disturbances and sensing things that are not real have been reported especially in children
• Faster heart beat
• Abnormal heart beat
• Widening of blood vessels
• Throat irritation
• Feeling sick
• Vomiting
• A dry, sore mouth
• Mouth irritation
• Muscle cramps.
Very rare: may affect up to 1 in 10,000 people
• Difficulty in sleeping
• Difficulty in breathing or wheezing
• Irregular heart beat, especially when used with beta-blockers
• Chest pain
• Itching skin.
Not known: frequency cannot be estimated from the available data
• Restriction of blood supply to the heart.
Using Airomir Autohaler may rarely lead to a build up of lactic acid or low potassium levels in your blood. Your doctor may wish you to have regular blood tests to check your blood potassium levels.
Although it is not known exactly how often this happens, some people may experience chest pain (due to heart problems such as angina). Tell your doctor as soon as possible if you develop these symptoms whilst receiving treatment with Airomir, but do not stop taking this medicine unless told to do so.
If any of the side effects continue for more than a few days, get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Airomir Autohaler device
Keep this medicine out of the sight and reach of children.
Store this inhaler below 30°C. Avoid storage in direct sunlight or heat. Protect from frost.
The metal canister is pressurised. Do not attempt to puncture it or burn it, even when empty.
Do not use Airomir Autohaler device after the expiry date that is stated on the outer packaging. The expiry date refers to the last day of that month.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
Airomir Autohaler device does not contain CFC’s; instead, the inhaler contains a hydrofluoroalkane (HFA-134a) as an inactive ingredient. Chlorofluorocarbons (CFC’s) have been shown to damage the ozone layer in the atmosphere. HFA’s have been developed as a replacement for CFC’s because they do not damage the ozone layer.
What Airomir Autohaler device contains:
• The active ingredient is salbutamol.
Each puff contains salbutamol sulfate equivalent to salbutamol 100 micrograms.
• The other ingredients are oleic acid, ethanol, propellant HFA-134a. Propellant HFA-134a is a new propellant which has been developed to replace chlorofluorocarbons (CFCs). This inhaler does not contain CFC propellants.
What Airomir Autohaler device looks like and contents of the pack:
• The Airomir Autohaler device is a breath actuated pressurised inhalation suspension.
• The inhaler contains 200 puffs.
Marketing Authorisation Holder and Manufacturer
The Marketing Authorisation holder is TEVA UK Limited, Brampton Road, Hampden Park, Eastbourne, East Sussex, BN22 9AG,
United Kingdom.
Airomir Autohaler devices are made by 3M Health Care Limited, Loughborough, Leicestershire LE11 1EP, England.
This leaflet was last revised: January 2016 PL 00289/1411
87783-E
620613162
Autohaler is a trademark of 3M and used under license
REG0068388
Version 5.4
Appro'
Page 2 of 3
This is a representation of an electronic record that was signed electronically and this
PAGE IS THE MANIFESTATION OF THE ELECTRONIC SIGNATURE
Teva Pharmaceuticals Europe B.V 1.3.2
mockup-pil-uk-pl-00289-1410-airomir-inhaler-100mcg-pressurised-inhalatio
n-suspension-salbutamol-sulfate
Approvals
|
Signed by |
Meaning of Signature |
Server Date |
|
Michael Silvester |
Regulatory Affairs Approval |
03-Mar-2016 01:16:22 PM |
REG0068388 Version 5.4 Approved Page 3 of 3