Algopain-Eze 140 Mg Medicated Plaster
Out of date information, search anotherPACKAGE LEAFLET: INFORMATION FOR THE USER
ALGOPAIN-Eze 140 mg medicated plaster (Diclofenac Sodium)
Read all of this leaflet carefully before you start using this medicine.
This medicine is available without prescription. However, you still need to use ALGOPAIN-Eze 140 mg medicated plaster carefully to get the best results from it.
• Keep this leaflet. Y ou may need to read it again.
• Ask your pharmacist if you need more information or advice.
• Y ou must contact a doctor if your symptoms worsen or do not improve after 3 days.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section4.
In this leaflet:
1. What ALGOPAIN-Eze 140 mg medicated plaster is and what it is used for
2. Before you use ALGOPAIN-Eze 140 mg medicated plaster
3. How to use ALGOPAIN-Eze 140 mg medicated plaster
4. Possible side effects
5. How to store ALGOPAIN-Eze 140 mg medicated plaster
6. Further information
1. What ALGOPAIN-Eze 140 mg medicated plaster is and what it is used for
Diclofenac sodium is a medicine that reduces pain. It belongs to the non-steroidal anti-inflammatory drug (NSAID) group.
For adults and adolescents aged 16 years and over:
ALGOPAIN-Eze 140 mg medicated plaster is used for short-term treatment in the local symptomatic treatment of pain associated with acute strains, sprains or bruises on the arms and legs as a result of injuries, e.g. sports injuries.
2. Before you use ALGOPAIN-Eze 140 mg medicated plaster
Do not use this medicine
• if you are hypersensitive (allergic) to diclofenac, propylene glycol, butylhydroxytoluene or any of the other ingredients of ALGOPAIN-Eze 140 mg medicated plaster. (See section 6 for a comprehensive list of ingredients).
• if you are hypersensitive (allergic) to any other non-steroidal anti-inflammatory drug (NSAID, e.g. aspirin, ibuprofen).
• if you developed asthma, swelling of the skin or swelling and irritation inside the nose after taking aspirin or other NSAID s.
• if you are suffering from an active peptic ulcer
• on injured skin (e.g. skin abrasions, cuts, burns), infected skin or eczema
• during the last three months of pregnancy
• in children and adolescents less than 16 years old
Take special care with this medicine
• if you suffer or have previously suffered from bronchial asthma or allergies, you may experience a bronchial muscle cramp (bronchospasm), which makes breathing difficult.
• if you get a skin rash, remove the medicated plaster immediately and stop the treatment.
• if you have kidney, heart or liver problems, or if you suffer or have previously suffered from a gastrointestinal ulcer, intestinal inflammation or a tendency to bleeding.
Side effects can be reduced by using the lowest effective dose for the shortest possible time.
IMPORTANT precautions:
• if symptoms persist for longer than 3 days or worsen,, you should talk to a doctor.
• do not use the medicated plaster on the eyes or internal body surfaces (e.g. nostrils, airways, ear canals) or allow it to come into contact with them.
• elderly patients should use this medicine with caution, as they are more likely to experience side effects.
• do not use if you are applying or taking other medicines containing diclofenac.
• do not use if you are applying or taking other NSAID painkillers or aspirin.
After taking off the medicated plaster avoid exposing the treated area to direct and solarium sunlight
for about one day in order to reduce the risk of sensitivity to light.
Children and adolescents
This medicine should not be used in children and adolescents aged 16 or below.
Using with other medicines
Please tell your doctor or pharmacist if you are taking/using or have recently taken/used any other medicines, including medicines obtained without a prescription. Provided that this medicine is used correctly, very little diclofenac is absorbed into the body, reducing the risk of interactions with other medicines.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before using any medicine. During the first six months of pregnancy, you should only use this medicine after speaking to your doctor. During the last three months of pregnancy, do not use this medicine, as there is an increased risk of complications for mother and baby (see “Do not use this medicine”). Very small quantities of diclofenac pass into the breast milk. As no adverse effects are known for the baby, there is no need to stop breast-feeding during short-term use of this medicine. However, this medicine should not be directly applied onto the breast area.
Driving and using machines
This medicine does not affect your ability to drive and use machines.
Important information about some of the ingredients of this medicine
Propylene glycol may cause skin irritation. Butylhydroxytoluene can cause local skin reactions (e.g. contact dermatitis), or irritation of the eyes and internal body surfaces (e.g. nostrils, airways, ear canals).
3. How to use ALGOPAIN-Eze 140 mg medicated plaster
Always use this medicine exactly as your doctor or pharmacist has told you to or according to this Package Leaflet. You should check with your doctor or pharmacist if you are not sure.
The usual dose is:
Attach one medicated plaster to the painful area twice daily, in the morning and the evening. The maximum total daily dose is 2 medicated plasters, even if there is more than one injured area to be treated. Treat only one painful area at once.
Children and adolescents
There are insufficient data on efficacy and safety for children and adolescents below 16 years of age.
Method of use
For application to the skin only. Do not take orally!
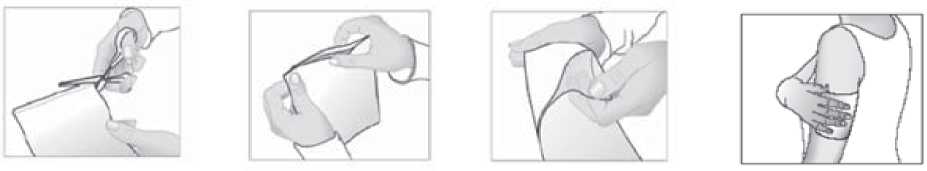
1. Cut open the bag containing the medicated plaster along the marking.
2. Take out one medicated plaster and carefully reseal the bag by pressing on the closure.
3. Remove the protective film from the adhesive surface of the medicated plaster.
4. Now apply the medicated plaster to the painful area.
If necessary, the medicated plaster can be held in place using an elastic net bandage.
Do not use the medicated plaster together with an air-tight (occlusive) bandage.
You should not cut the medicated plaster.
Used plasters should be folded in half, with the sticky side inwards.
Duration of use
Do not use this medicine for longer than 3 days without your doctor’s advice. The use of this medicine for a longer time must be discussed with a doctor and should not exceed 7 days.
If you have the impression that the effect of this medicine is too strong or too weak, please talk to your doctor or pharmacist.
If you use more ALGOPAIN-Eze 140 mg medicated plaster than you should
Please tell your doctor if marked side effects occur after incorrect use of this medicine or accidental overdose (e.g. in children). They will be able to advise you of any further measures which may be necessary, depending on the severity of poisoning.
If you forget to use ALGOPAIN-Eze 140 mg medicated plaster
Do not use a double dose to make up for a forgotten dose. If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following frequency conventions are used in the evaluation of side effects:
Tell your doctor immediately and stop using the plaster, if you notice any of the following:
• sudden itchy rash (hives);
• swelling of the hands, feet, ankles, face, lips, mouth or throat;
• difficulty breathing;
drop in blood pressure or weakness.
You may experience the following side effects:
Common (less than 1 in 10 persons treated):
Local skin reactions, such as skin reddening, a burning sensation, itching, inflamed skin redness, skin rash, sometimes with pustules or wheals.
Uncommon (less than 1 in 100 persons treated):
Hypersensitivity reactions or local allergic reactions (contact dermatitis). In patients who have applied active substances to the skin belonging to the same drug class as diclofenac, there have been isolated reports of generalised skin rash, hypersensitivity reactions, such as swelling of the skin and mucous membranes and anaphylactic-type reactions with acute circulatory regulation disorders and sensitivity to light.
Absorption of diclofenac into the body via the skin is very low compared with the concentration of the active substance in the blood following oral intake of diclofenac. The probability of side effects occurring in the body as a whole (such as gastrointestinal or kidney disturbances or breathing difficulties) is therefore very low. But if you do get these or if any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store ALGOPAIN-Eze 140 mg medicated plaster Keep out of the reach and sight of children.
Do not use this medicine after the expiry date which is stated on the outer carton and the bag after EXP. The expiry date refers to the last day of that month.
Do not store above 25 °C. Store in the original package in order to prevent the patches from drying out and to protect them from light.Keep the bag tightly closed in order to prevent the patches from drying out.
Can be stored for 4 months after first opening of a bag.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Further information
What ALGOPAIN-Eze 140 mg medicated plaster contains
The active substance is diclofenac sodium. Each medicated plaster contains 140 mg diclofenac sodium.
The other ingredients are glycerol; propylene glycol (E1520); diisopropyl adipate; sorbitol, liquid (crystallising) (E420); carmellose sodium; polyacrylic acid sodium salt; basic butylated methacrylate copolymer; disodium edetate; sodium sulphite, anhydrous (E221); butylhydroxytoluene (E321); aluminium-potassium-bis(sulphate); silica, colloidal anhydrous; light kaolin (natural); macrogol lauryl ether (9 EO units); levomenthol; tartaric acid; purified water; unwoven polyester support; polypropylene protective film.
What ALGOPAIN-Eze 140 mg medicated plaster looks like and contents of the pack
ALGOPAIN-Eze 140 mg medicated plaster is a 10 x 14 cm medicated plaster with a white to light brown paste spread as an even layer onto unwoven support and with a detachable protective film.
ALGOPAIN-Eze 140 mg medicated plaster is available in packs with 2, 5, 10 or 14 medicated plasters in resealable bags each containing 2 or 5 medicated plasters.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer Marketing Authorisation Holder:
ratiopharm GmbH, Graf-Arco-Strasse 3, D-89079 Ulm, Germany.
Manufacturer:
Merckle GmbH, Ludwig-Merckle-StraBe 3, 89143 Blaubeuren, Germany.
This medicinal product is authorised in the Member States of the EEA under the following
names:
Austria:
Belgium:
Czech Republic:
Germany:
Hungary:
Italy:
Slovakia:
Spain:
United Kingdom:
Dolostrip 140 mg wirkstoffhaltiges Pflaster Kinespir Patch 140 mg pleister Olfen 140 mg lecive naplasti Diclofenac-ratiopharm Schmerzpflaster Algoplast-ratiopharm 140 mg gyogyszeres tapasz Diclofenac Pharmentis 140 mg cerotti medicati Diclobene 140 mg
ratioparch 140 mg apositos adhesivos medicamentosos ALGOPAIN-Eze 140 mg medicated plaster
For a large print, audio, Braille or CD-rom version of this patient information leaflet, phone 02392 313592.
This leaflet was last revised in October 2014.