Almus Decongestant Nasal Spray
Out of date information, search another

69.53 mm
|
: WFM | ||
|
BLOCKED NOSE |
E E | |
|
RELIEF 0.05% w/v; |
S3 S | |
|
\ Nasal Spray ; |
\ Oxymetazoline \ Hydrochloride
WSSV2
Use by the date shown above ] i/How to use this medicine ,
Read and retain carton for i full instructions.
Adults and children of 12 , years and over: One or two r
' sprays into each nostril every'
! 6-8 hours. PL 00014/029^'
' The Boots Company PLC i 'Nottingham NG2 3AA /
Trident Reference No: BTC1 76369 Zen Ref: TR922110
|
Category: |
Healthcare |
|
Sub-Category: |
Cough Cold |
|
Brand: |
Core |
|
Pack Type: |
Bottle |
|
Variant: |
Blocked Nose Relief 0.05% w/v Nasal Spray 22 ml |
|
Action: |
c |
|
Date: |
26/02/15 |
|
Country: |
UK |
|
Component Code: |
WSSV2 |
|
Item Code: |
30-07-316 |
|
CAD Ref No: |
n/a |
|
Printer: |
N/A |
|
Substrate: |
White Plastic |
|
Barcode Type: |
N/A |
|
Barcode Number: |
N/A |
|
Magnification: |
N/A |
|
Barcode Truncated By: (smallest bar) |
N/A |
|
Edgemark Position: |
n/a |
|
Pharmacode No/NE: |
N/A |
Technical ft Non Printing Items Cutter | Guides
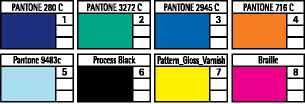
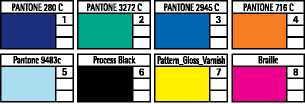
Colours
|
PANTONE 280 C | |
|
= | |
|
Main Headings: |
N/A |
|
Sub Headings: |
N/A |
|
Body Copy: |
7pt |
|
PACK MOCK LIP | |||
|
Product Name: |
Blocked Nose Relief 0.05% w/v Nasal | ||
|
Spray | |||
|
Product Licence No.: |
00014/0292 | ||
|
Wording Ref: |
MHRA approved v1 dated 153.11 (BTC79756 Action C) | ||
|
Status: |
Internally approved | ||
|
Pack Details: |
PPr bottle with polyethylene plug and elongated | ||
|
nozzle dip tube fitted with wadless polyethylene | |||
|
cap that fits the elongated nozzle | |||
|
Pack Size: |
22 ml | ||
|
Version No. |
Date Issued |
Reason For Change | |
|
1 |
24/02/15 |
Redesign | |
|
Category: |
Healthcare |
|
Sub-Category: |
Cough Cold |
|
Brand: |
Core |
|
Pack Type: |
Bottle |
|
Variant: |
Blocked Nose Relief 0.05% w/v Nasal Spray 22 ml |
|
Action: |
c |
|
Date: |
26/02/15 |
|
Country: |
UK |
Component Code: WSSV2
Item Code: 30-07-31 6
|
Barcode Type: |
N/A |
|
Barcode Number: |
N/A |
|
Magnification: |
N/A |
|
Barcode Truncated By: (smallest bar) |
N/A |
|
Edgemark Position: |
n/a |
|
Pharmacode No/NE: |
N/A |
|
Main Headings: |
N/A |
|
Sub Headings: |
N/A |
|
Body Copy: |
7pt |
|
PACK MOCK LIP | |||
|
Product Name: |
Blocked Nose Relief 0.05% w/v Nasal | ||
|
Spray | |||
|
Product Licence No.: |
00014/0292 | ||
|
Wording Ref: |
MHRA approved v1 dated 153.11 (BTC79756 Action C) | ||
|
Status: |
Internally approved | ||
|
Pack Details: |
PPr bottle with polyethylene plug and elongated | ||
|
nozzle dip tube fitted with wadless polyethylene | |||
|
cap that fits the elongated nozzle | |||
|
Pack Size: |
22 ml | ||
|
Version No. |
Date Issued |
Reason For Change | |
|
1 |
24/02/15 |
Redesign | |


BLOCKED NOSE RELIEF 0.05% w/v; \ Nasal Spray \ Oxymetazoline \ Hydrochloride
Use by the date shown above ] /How to use this medicine ,
Read and retain carton for i
full instructions. 1
i Adults and children of 12 ,
' years and over: One or two r
' sprays into each nostril every'
! 6-8 hours. PL 00014/029^'
' The Boots Company PLC i 'Nottingham NG2 3AA /
CAD Ref No: n/a
Printer:
Substrate: White Plastic
Technical ft Non Printing Items Cutter | Guides
Colours
|
PANTONE 280 C | |
|
= | |
176 mm
How to store this medicine
Do not store above 25<IC, Keep all medicines out of the sight and reach of children.
Use by the date on the side panel.
Active ingredient
This spray contains Oxymetazoline Hydrochloride 0.05% w/v.
Also contains: purified water, disodium phosphate, monosodium phosphate, ethanol, methyl hydroxybenzoate {E218), cetrimide, levomenthol, camphor racemic, eucalyptol.
fads IBSEN
A/M%SO'OR I13U 3S0N 03)10010
Read all of this carton for full instructions.
What this medicine is for
This medicine contains a decongestant, which acts to relieve nasal congestion. It can be used for fast relief of stuffy noses due to head colds and hayfever.
Before you use this medicine
If you are allergic to any of the ingredients If you have heart or blood vessel disease
• If you are taking monoamine oxidase inhibitors (for depression) or have taken them in the last 14 days
• If you have an overactive thyroid
• If you have glaucoma
• If you are a man with prostate problems
f Talk to your pharmacist or doctor:
• If you are pregnant or breastfeeding
Methyl hydroxybenzoate (E218) may cause allergic reactions (possibly delayed).
How to use this medicine
Check the seal is not broken before first use.
If it is, do not use the medicine.
|
Age |
How much |
How often I |
|
Adults and children of 12 years and over |
One or two sprays into each nostril |
Every 6 to 8 i hours. i |
For use in the nose only.
Do not use for children under 12 years.
Do not use more than the amount recommended above.
Do not use this medicine for more than 7 days unless your doctor tells you to.
If symptoms do not go away talk to your doctor, tfyqujjse too mucfc Talkjoa doctor.__
BLOCKED NOSE BELIEF 0.05% w/\v Nasal Spray
Oxymetazoline Hydrochloride
mmm
' Rapid relief for blocked nose
PL 00014/0292 Text prepared 02/15 Manufactured by BCM Ltd Nottingham NG2 3AA for the MAH: The Boots Company PLC Nottingham NG2 3AA
If you need more advice ask your pharmacist.
Possible side effects
Most people will not have problems, but some may get some of these:
• Irritation to the nose Dry mouth and throat Return of your symptoms if used for a long time
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed on this carton, You can also report side effects directly via the Yellow Card Scheme at: www.mhraaw.uMdtawcard. By reporting side effects you can help provide more information on the safety of this medicine.
KUXV2
_l_^r-
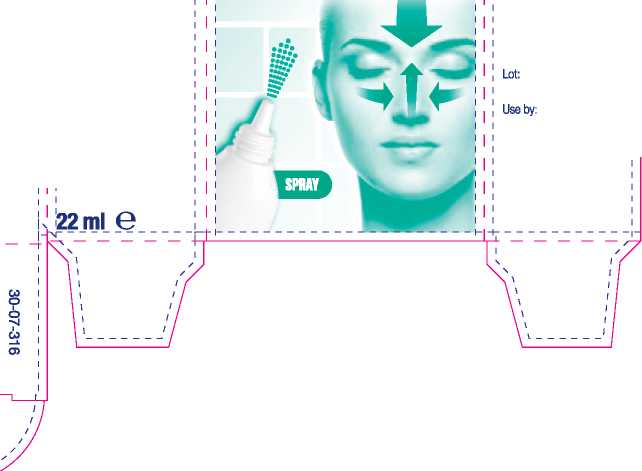
22 ml
|
Main Headings: |
8pt |
|
Sub Headings: |
7pt |
|
Body Copy: |
6pt |
|
PACK MOCK UP | ||
|
Product Name: Blocked Nose Relief 0.05% w/v Nasal Spray Product Licence No.: 00014/0292 Wording Ref: MHRAApproved vl 15/03/2011 (BTC69436v C] Status: 1 nterna 1 ly a p p ro ved Pa ck Deta i 1 s: PPr bottle with polyethylene plugandelongated nozzle dip tube fitted with wadless polyethylene cap that fits the elongated nozzle Pack Size: 22 ml | ||
|
Version No. |
Date Issued |
Reason For Change |
|
1 |
09/02/15 |
Redesign. Add yellow card warning and BCM manufacturer address |
|
Trident Reference No: BTC176356 | |
|
Zen Ref: |
TR920580 |
|
Category: |
Healthcare |
|
Sub-Category: |
Cough Cold |
|
Brand: |
Core |
|
Pack Type: |
Carton |
|
Variant: |
Blocked Nose Relief 0.05% w/v Nasal Spray 22 ml |
|
Action: |
B |
|
Date: |
16/02/15 |
|
Country: |
UK |
|
Component Code: |
KWXV2 |
|
Item Code: |
30-07-316 |
|
CAD Ref No: |
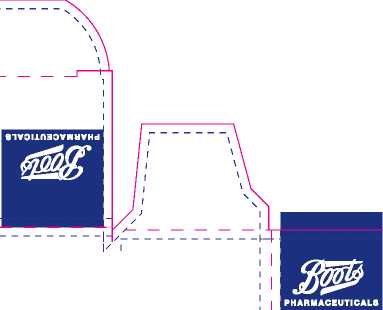
47 mm x 27 mm x98 mm |
|
Printer: |
N/A |
|
Substrate: |
White Carton Board |
|
Barcode Type: |
EAN 13 |
|
Barcode Number: |
5045093007319 |
|
Magnification: |
80% |
|
Barcode Truncated By: (smallest bar) |
2.3 mm |
|
Edgemark Position: |
4 |
|
Pharmacode No/NE: |
N/A |
Technical ft Non Printing Items Cutter | Guides
Colours
176 mm
|
Main Headings: |
8pt |
|
Sub Headings: |
7pt |
|
Body Copy: |
6pt |
|
Trident Reference No: BTC176356 | |
|
Zen Ref: |
TR920580 |
|
Category: |
Healthcare |
|
Sub-Category: |
Cough Cold |
|
Brand: |
Core |
|
Pack Type: |
Carton |
|
Variant: |
Blocked Nose Relief 0.05% w/v Nasal Spray 22 ml |
|
Action: |
B |
|
Date: |
16/02/15 |
|
Country: |
UK |
|
Component Code: |
KWXV2 |
|
Item Code: |
30-07-316 |
|
CAD Ref No: |
47 mm x 27 mm x98 mm |
|
Printer: |
N/A |
|
Substrate: |
White Carton Board |
|
Barcode Type: |
EAN 13 |
|
Barcode Number: |
5045093007319 |
|
Magnification: |
80% |
|
Barcode Truncated By: (smallest bar) |
2.3 mm |
|
Edgemark Position: |
4 |
|
Pharmacode No/NE: |
N/A |
Technical ft Non Printing Items Cutter | Guides
BRAILLE CONVERSION - English Alphabet
|
• |
• |
• |
• • |
• |
• |
• |
• |
• |
• | |||
|
• |
• |
• |
• |
• |
• |
• | ||||||
|
• • |
• |
• |
• |
• |
• |
• |
• |
• |
• • | |||
|
• | ||||||||||||
|
• |
• |
• |
• |
• | ||||||||
|
• |
• |
• |
• |
• • |
• |
• |
• |
• | ||||
|
• |
• • |
• • |
• • |
• |
• • |
• |
• • |
• |
• | |||
|
• • |
• |
• |
• |
• |
• |
• • | ||||||
|
• • |
• |
• |
• |
• |
• |
• • |
• |
• |
• • | |||
|
• |
• |
• |
• |
• |
• • |
• | ||||||
|
• |
• |
• |
• |
• |
• |
• • |
|
PACK MOCK UP | ||
|
Product Name: Blocked Nose Relief 0.05% w/v Nasal Spray Product Licence No.: 00014/0292 Wording Ref: MHRAApproved vl 15/03/2011 (BTC69436v C] Status: I nterna I ly a p p ro ved Pa ck Deta i I s: PPr bottle with polyethylene plugandelongated nozzle dip tube fitted with wadless polyethylene cap that fits the elongated nozzle Pack Size: 22 ml | ||
|
Version No. |
Date Issued |
Reason For Change |
|
1 |
09/02/15 |
Redesign. Add yellow card warning and BCM manufacturer address |
Colours
Boots Blocked Nose Relief Nus5!T0.05 o/o w/v Nasal Spray
176 mm
How to store this medicine
Do not store above 25<IC, Keep all medicines out of the sight and reach of children.
Use by the date on the side panel^ ^ ^
• •
Active ingredient
This spraygpntains Oxymetazolina HydrochlojxjeJ.^% w/v.
Also confers: purified water, disoffuB • phosphat^nonosodium phosphate, efeaml, methyl hydroxytenzoate {E218), cetrimide, levomenthol, camphor racemic, eucatyptol.
fads IBSEN
A/M%SO'OR I13U 3S0N 03)10010
Read all of this carton for full instructions.
What this medicine is for
This medicine contains a decongestant, which acts to relieveJaSl congestion. It can be usafiorfast relief of sffrffy noses due to head cMl^ra hayfever.
Before yoff use this mediJne
If you are allergic to#iy of the in|feiflferA
• If you h4Vb heCt or flldld vessel disease
• If you ar^taking monormine oxjgaM jahibitors
(for depr^ion) or ha^taken them in Je last 14 days
• If you have an overactive thyroid
• If you have^laucon#
• If you arffa m§h with pAstate problems
f Talk to your pharmacist or &>ctor:
• If you ar^rl|rwit or A^tAfedin<A * •
Methyl hydroxybenzoate P?18) may cAise allergic
How to use thiMetficine
Check thgseal is not broken before fir#ui If it is, do nofciA the medicine. •
|
Age | ||
|
Adults and children oW2 years agt ov^ |
ftl 111 |
CO _g CD fil |
For use in thgnose only. • % g
Do not ug for qtyldregpndeg^ 2 yearqg Do not use more than theamount recommended above* * * * *
Do not u* *s medicme for* ore than 7 frays unless your doctor tells you to.
If symptoms do not go away talk to your doctor, tfyqujjse too muchjjalktoa doctor.__
BLOCKED NOSE BELIEF 0.05% w/\v Nasal Spray
Oxymetazoline Hydrochloride
mmm
' Rapid relief for blocked nose
PL 00014/0292 Text prepdAd 02/15 Manufactugd by BCM Ltd Nottingham N02gAA for the MAH: The Boots Company ELC Nottingham NG2 3AA*
If you neec#n#e advice ask your pfcrmaget.
5 045093007319>
Possible side effects
Most people will not have problems, but some may get some of these:
• Irritation to the nose Dry mouth and throat Return of your symptoms if used for a long time
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed on this carton, You can also report side effects directly via the Yellow Card Scheme at: www.mhraaw.uMdtawcard. By reporting side effects you can help provide more information on the safety of this medicine.
KUXVE
|
Main Headings: |
8pt |
|
Sub Headings: |
7pt |
|
Body Copy: |
6pt |
|
Trident Reference No: BTC176356 | |
|
Zen Ref: |
TR920580 |
|
Category: |
Healthcare |
|
Sub-Category: |
Cough Cold |
|
Brand: |
Core |
|
Pack Type: |
Carton |
|
Variant: |
Blocked Nose Relief 0.05% w/v Nasal Spray 22 ml |
|
Action: |
B |
|
Date: |
16/02/15 |
|
Country: |
UK |
|
Component Code: |
KWXV2 |
|
Item Code: |
30-07-316 |
|
CAD Ref No: |
47 mm x 27 mm x98 mm |
|
Printer: |
N/A |
|
Substrate: |
White Carton Board |
|
Barcode Type: |
EAN 13 |
|
Barcode Number: |
5045093007319 |
|
Magnification: |
80% |
|
Barcode Truncated By: (smallest bar) |
2.3 mm |
|
Edgemark Position: |
4 |
|
Pharmacode No/NE: |
N/A |
Technical ft Non Printing Items Cutter | Guides
BRAILLE CONVERSION - English Alphabet
|
• |
• |
• |
• • |
• |
• |
• |
• |
• |
• | |||
|
• |
• |
• |
• |
• |
• |
• | ||||||
|
• • |
• |
• |
• |
• |
• |
• |
• |
• |
• • | |||
|
• | ||||||||||||
|
• |
• |
• |
• |
• | ||||||||
|
• |
• |
• |
• |
• • |
• |
• |
• |
• | ||||
|
• |
• • |
• • |
• • |
• |
• • |
• |
• • |
• |
• | |||
|
• • |
• |
• |
• |
• |
• |
• • | ||||||
|
• • |
• |
• |
• |
• |
• |
• • |
• |
• |
• • | |||
|
• |
• |
• |
• |
• |
• • |
• | ||||||
|
• |
• |
• |
• |
• |
• |
• • |
|
PACK MOCK UP | ||
|
Product Name: Blocked Nose Relief 0.05% w/v Nasal Spray Product Licence No.: 00014/0292 Wording Ref: MHRAApproved vl 15/03/2011 (BTC69436v C] Status: I nterna I ly a p p ro ved Pa ck Deta i I s: PPr bottle with polyethylene plugandelongated nozzle dip tube fitted with wadless polyethylene cap that fits the elongated nozzle Pack Size: 22 ml | ||
|
Version No. |
Date Issued |
Reason For Change |
|
1 |
09/02/15 |
Redesign. Add yellow card warning and BCM manufacturer address |
Colours
Boots Blocked Nose Relief Nus5!T0.05 o/o w/v Nasal Spray