Alphanate Powder For Injection
RTA#81 11 7/8
RTA#81 11 7/8
G
N/d
3a oo yva
GRIFOLS
PACKAGE LEAFLET: INFORMATION FOR THE USER
8/9 8
Alphanate®
Powder and solvent for solution for injection
G
B
B
Human coagulation factor VIII and von Willebrand
factor complex
Read all of this leaflet carefully before you start
using this medicine because it contains important
information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Alphanate® is and what it is used for
2. What you need to know before you use Alphanate®
3. How to use Alphanate®
4. Possible side effects
5. How to store Alphanate®
6. Contents of the pack and other information
1. WHAT ALPHANATE® IS AND WHAT IT IS USED FOR
Alphanate® is supplied as a powder for solution for injection containing approximately 250, 500, 1000 or 1500 IU of human coagulation factor VIII and 300, 600, 1200 or 1800 IU of human von Willebrand factor per vial (Note IU stands for international unit, a standard measure of activity).
Once reconstituted with the appropriate amount of solvent (water for injections), each vial contains approximately 50, 100 or 150 IU of FVIII/ml and 60, 120 or 180 IU VWF/ml.
Alphanate® is one of the group of medicines called clotting factors.
Alphanate® is used for the treatment and prevention of bleeding in patients with haemophilia A (congenital factor VIII deficiency).
Alphanate® is used for the treatment and prevention of bleeding (haemorrhage) or surgical bleeding in von Willebrand disease (VWD), when desmopressin (DDAVP) treatment alone is ineffective or contraindicated.
This product may be used in the management of acquired factor VIII deficiency.
2. WHAT YOU NEED TO KNOW BEFORE YOU USE ALPHANATE®
Do not use Alphanate®
• If you are allergic to human coagulation factor VIII and von Willebrand factor complex or any of the other ingredients of this medicine (listed in section 6).
• If you have not been fully trained how to inject yourself by your doctor or haemophilia nurse.
If you want more detailed information then ask your doctor.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using Alphanate®.
• Rarely you may have an anaphylactic reaction (a sudden severe allergic reaction) such as rash, tightness of the chest, dizziness, vertigo or nausea, or feeling dizzy when you are standing. If these symptoms occur, you must stop using the product immediately and contact your doctor.
• Your doctor should perform some tests to make sure that the dosage of Alphanate® you are receiving is enough to achieve and maintain appropriate factor VIII levels and thus stop any bleeding.
• If your bleeding is not controlled with Alphanate®, consult your doctor immediately. You may have developed factor VIII inhibitors, these are antibodies which block the clotting effect of factor VIII. Your doctor will perform some tests to confirm whether the inhibitors are present in your blood.
• During the treatment of the von Willebrand disease, there is a chance you may develop blood clots, particularly if you have known clinical risk factors. Therefore, your doctor should perform some tests to detect early signs of blood clots and recommend the treatment if necessary.
• Patients with von Willebrand disease, especially type 3 patients, may develop antibodies (inhibitors) to von Willebrand factor. Your doctor may perform more blood tests to confirm whether the inhibitors are present in your blood.
When medicines are made from human blood or plasma, certain measures are put in place to prevent infections being passed on to patients. These include:
- careful selection of blood and plasma donors to make sure those at risk of carrying infections are excluded,
- the testing of each donation and pools of plasma for signs of virus/infections,
- the inclusion of steps in the processing of the blood or plasma that can inactivate or remove viruses.
Despite these measures, when medicines prepared from human blood or plasma are administered, the possibility of passing on infection cannot be totally excluded. This also applies to any unknown or emerging viruses or other types of infections.
The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus and hepatitis C virus. The measures taken may be of limited value against non-enveloped viruses such as hepatitis A virus and parvovirus B19. Parvovirus B19 infection may be serious for pregnant women (foetal infection) and for individuals whose immune system is depressed or who have some types of anaemia (e.g. sickle cell disease or haemolytic anaemia).
Your doctor may recommend that you consider vaccination against hepatitis A and B if you regularly/repeatedly receive human plasma-derived factor VIII products.
It is strongly recommended that every time you receive a dose of Alphanate® the name and batch number of the product are recorded in order to maintain a record of the batches used.
See also section 4.
Other medicines and Alphanate®
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
No interactions of Alphanate® with other medicines are known.
Do not mix Alphanate® with any other medicines that you receive by injection.
Pregnancy and breast-feeding
Based on the rare occurrence of haemophilia A in women, experience regarding the use of FVIII/VWF complex during pregnancy and breast-feeding is not available.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Alphanate® has little or none effect on your ability to drive and use machines.
Sodium content
The residual content of sodium in Alphanate®, arising from the manufacturing process, does not exceed 23 mg (1 mmol) per vial. This is equivalent to 1.15% of the recommended maximum daily intake of sodium for an adult. However, depending on the body weight of the patient and the posology, the patient may receive more than one vial.
3. HOW TO USE ALPHANATE®
Reconstitute the product as described at the end of this leaflet (please see pictograms on the back of the leaflet). The product must be given by intravenous route. The administration rate should be 3 ml/min and never more than 10 ml/min to avoid undesirable side effects. Inject Alphanate® immediately after reconstitution.
The amount of Alphanate® you should use depends on many factors, such as your weight, your clinical status and the type and severity of bleeding. Your doctor will calculate the dose, the frequency and the intervals of administration of Alphanate® in order to reach the necessary level of factor VIII or von Willebrand factor in your blood.
Your doctor will tell you the duration of your treatment with Alphanate®.
You will be given full training before using Alphanate® without the hospital supervision. Please refer to all your training materials or contact your local haemophilia centre for more information. You should not self-administer Alphanate® alone: always have a responsible adult present.
Use in children and adolescents
There are insufficient data from clinical trials to recommend the use of Alphanate® in children less than 6 years of age for the authorised indications.
If you use more Alphanate® than you should
No cases of overdose with Alphanate® have been reported. However, if you have used Alphanate® more than required, consult your doctor or pharmacist immediately.
If you forget to use Alphanate®
Proceed immediately with the following dose and continue at regular intervals as directed by your doctor. Do not take a double dose to make up for a forgotten dose.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
On rare occasions, you may have some of the following side effects after the administration of Alphanate®. Please contact your doctor immediately if you experience:
• itching, local reactions at the injection site (e.g. burning and transitory reddening)
8/9 8
• allergic reactions (e.g. tightness of the chest/ feeling unwell, dizziness, nausea and slight drop of blood pressure than can make you feel dizzy when you are standing)
• fever
• faster heart beat (tachycardia)
Occasionally an anaphylactic shock may occur. If you observe any of the following symptoms during the injection/perfusion, interrupt the injection/ perfusion and contact your doctor immediately:
• tightness of the chest/feeling unwell
• dizziness
• slight hypotension (slight drop of blood pressure with dizziness when you are standing)
• nausea
Allergic reactions to the components of the product cannot be totally excluded. The formation of neutralising antibodies of factor VIII (inhibitors) is a well-known complication in the treatment of patients with haemophilia A. Study results show that first time users of factor VIII are mainly affected. You will be carefully monitored for the development of these inhibitors.
There is a chance of blood clots, particularly if you have known clinical risk factors.
Reporting of side effects If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via Yellow Card Scheme, Website: https://yellowcard.mhra.gov.uk. By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE ALPHANATE®
Keep this medicine out of the sight and reach of children.
Do not store above 30 °C. Do not freeze. Protect from light.
Do not use this medicine after expiry date which is stated on the vial label and carton after EXP.
Do not use this medicine if you notice the solution is cloudy or has deposits. Generally the solution is clear or slightly opalescent. If the solution is discoloured or cloudy discard it.
Alphanate® should be administered immediately after reconstitution
Any unused product or waste material should be disposed of in accordance with local requirements. Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Alphanate® contains
The active substance is human coagulation factor VIII and von Willebrand factor complex.
Alphanate® is presented as powder for solution for injection containing approximately 250, 500, 1000 or 1500 IU human coagulation FVIII and 300, 600, 1200 or 1800 IU of human von Willebrand factor per vial. The product is reconstituted with 5 ml of water for injections for the presentations of 250 and 500 IU or 10 ml of water for injections for the presentations of 1000 and 1500 IU.
The other ingredients are albumin, histidine and arginine.
What Alphanate® looks like and contents of the pack
Vial containing white or pale yellow powder and syringe with water for injections (solvent). Presentations:
Alphanate® 250 IU (250 IU/5 ml)
Alphanate® 500 IU (500 IU/5 ml)
Alphanate® 1000 IU (1000 IU/10 ml)
Alphanate® 1500 IU (1500 IU/10 ml) PL 12930/0015 Water for Injections PL 4447/0016
Pack size: 1 lyophilised vial, 1 syringe pre-filled with solvent and accessories (vial adaptor, filter, 2 alcohol swabs and butterfly needle).
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder and Manufacturer:
Instituto Grifols, S.A.
Can Guasc, 2 - Parets del Valles 08150 Barcelona - SPAIN
Distributed by:
Grifols UK Ltd.
Byron House
Cambridge Business Park Cambridge CB4 0WZ
This leaflet was last revised in April 2016
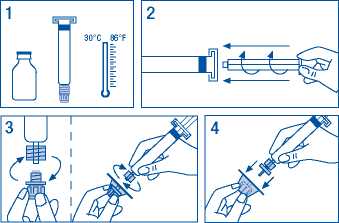
Instructions for use/handling
Follow these instructions unless otherwise indicated
by your doctor.
Left-over product must never be kept for later use, nor
stored in a refrigerator.
To prepare the solution:
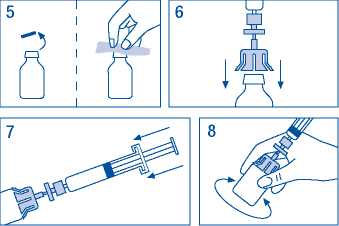
1. Warm the vial and syringe but not above 30 °C.
2. Attach plunger to syringe containing solvent.
3. Remove filter from packaging. Remove cap from syringe tip and attach syringe to filter.
4. Remove vial adaptor from packaging and attach to syringe and filter.
5. Remove cap from vial and wipe stopper with swabs provided.
6. Pierce vial stopper with adaptor needle.
7. Transfer all solvent from syringe to vial.
8. Gently shake vial until all product is dissolved. As with other parenteral solutions, do not use if product is not properly dissolved or particles are visible.
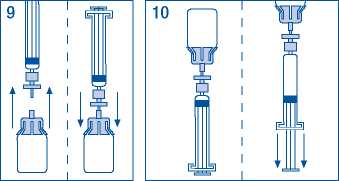
9. Briefly separate the syringe/filter from vial/ adaptor, to release the vacuum.
10. Turn the vial upside down and draw the solution into the syringe.
11. Prepare injection site, separate syringe and inject product using the butterfly needle provided. Injection rate should be 3 ml/min into a vein and never more than 10 ml/min to avoid vasomotor reactions.
Do not re-use administration sets.
11
r

P-2
