Alvesco Takeda 160 Inhaler
Takeda
o°o°/AIVCSCO® Takeda
This medicine is not suitable for use in an acute attack of breathlessness. For quick relief from such an attack, use only your Reliever inhaler.
PACKAGE LEAFLET: INFORMATION FOR THE USER
Alvesco Takeda 40, 80 and 160 micrograms pressurised inhalation, solution Ciclesonide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
See section 4.
What is in this leaflet:
1. What Alvesco Takeda is and what it is used for
2. What you need to know before you use Alvesco Takeda
3. How to use Alvesco Takeda
4. Possible side effects
5. How to store Alvesco Takeda
6. Contents of the pack and other information
1. WHAT ALVESCO TAKEDA IS AND WHAT IT IS USED FOR
What Alvesco Takeda is:
Alvesco Takeda is a clear and colourless aerosol spray for you to breathe in through your mouth and into your lungs. It is a Preventer medication (corticosteroid) that has to be taken every day and which becomes active only after it has been inhaled into your lungs.
The active ingredient in this medicine is ciclesonide. (For the other ingredients, see Section 6).
What Alvesco Takeda is used for:
This medicine is prescribed to control persistent asthma in adult and adolescent patients (12 years old and more).
It helps you to breathe more easily by decreasing the symptoms of your asthma and by lessening the chances of an asthma attack. The effect builds up over a period of time, so this medicine needs to be taken every day, even when you are feeling well.
2. WHAT YOU NEED TO KNOW BEFORE YOU USE ALVESCO TAKEDA
Do not use Alvesco Takeda
if you are allergic (hypersensitive) to ciclesonide or any of the other ingredients of this medicine. (listed in section 6).
Take special care with Alvesco Takeda
• Before beginning treatment with this medicine, please tell your doctor if:
you have ever been treated, or are currently being treated, for pulmonary tuberculosis (TB), fungal, viral or bacterial infections.
Check with your doctor if you are not sure. It is important to make sure that Alvesco Takeda is the right medicine for you.
During your treatment with Alvesco Takeda contact your doctor immediately if:
• breathing becomes difficult and your symptoms, coughing, breathlessness, wheezing, tightness in the chest, increasing noises (rhonchi) or other symptoms of narrowing of the airways are getting worse.
(You should use your Reliever inhaler which will normally lead quickly to an improvement.)
• you are waking up at night with your symptoms.
• you are not getting relief from using your Reliever inhaler.
Your doctor will decide on your further treatment.
Specific patient groups
Patients with severe asthma are at risk of acute asthma attacks. For such patients the doctor will carry out regular thorough asthma control checks, including a lung function test.
Patients who are already taking corticosteroid tablets:
Alvesco Takeda can be used to replace your tablets, or to reduce the number of tablets you need to take. Please follow your doctor's instructions carefully.
- This will start about a week after you begin your Alvesco Takeda inhalations.
- The number of tablets you take will be reduced with caution over a period of time.
- During this period you may sometimes suffer from a general feeling of being unwell.
- In spite of this, it is important to continue both with your Alvesco Takeda inhalations and with slowly reducing the number of tablets you take.
- If you get serious symptoms such as nausea (feeling sick), vomiting (being sick), diarrhoea or a high temperature, contact your doctor.
- This process may sometimes reveal minor allergies such as rhinitis (inflammation of the inside of the nose) or eczema (itchy, reddening skin).
- If you have changed over from tablets, you will continue for a time to be at risk of reduced adrenal function, which is related to the corticosteroid tablets you take. The symptoms of reduced adrenal function (e.g. dizziness, fainting, nausea, loss of appetite, moodiness, decrease in body hair, inability to cope with stress, weakness, headaches, memory problems, allergies, food cravings, and blood sugar disorders) may also continue for some time.
- You may also need to see a specialist to determine the extent of the reduction in adrenal function.
- Your doctor will also do regular checks on your adrenal function.
- During periods of stress, for example, having an operation, worsening asthma attacks, it is possible that you will need extra corticosteroid tablets. If so, you must carry a steroid warning card which says so.
Patients with liver or kidney disorders
There is no need to adjust the dose of ciclesonide if you have liver or kidney problems. If you suffer from a severe liver condition, your doctor will check you more carefully for possible side effects resulting from disturbance of normal steroid production.
Children below 12 years of age:
This medicine is not recommended for children below the age of 12 because of a lack of information about its possible effects.
Other medicines and Alvesco Takeda
Please inform your doctor before using Alvesco Takeda, if you are currently being treated for any fungal or viral infections with medicine containing:
- ketoconazole,
- itraconazole,
- ritonavir,
- nelfinavir.
These may intensify the action of Alvesco Takeda so that the probability of side effects cannot be completely ruled out.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Using this medicine with food and drink
There is no interaction between Alvesco Takeda and food and drink.
Pregnancy, breast feeding and fertility
Tell your doctor if you are pregnant, want to become pregnant or are breast-feeding.
- Because there is not enough information about the effects of Alvesco Takeda on pregnant women, your doctor will discuss with you the risks and benefits of using Alvesco Takeda.
- Ciclesonide (the active ingredient in Alvesco Takeda) may be taken during pregnancy only when the possible benefits to the mother justify the possible risk to the developing baby. If your doctor decides that you can continue using Alvesco Takeda, the smallest possible dose of ciclesonide will be used to maintain asthma control.
- The adrenal function will be carefully monitored in children of mothers who received corticosteroids during pregnancy.
- Talk to your doctor if you want to use Alvesco Takeda during breast-feeding.
- It is not known whether inhaled ciclesonide passes into the breast milk in humans.
- Prescribing Alvesco Takeda to women who are breast feeding will therefore only be considered if the expected benefit to the mother outweighs the possible risk to the child.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Alvesco Takeda and its ingredients have no or negligible effects on the ability to drive or to use machinery.
3. HOW TO USE ALVESCO TAKEDA
Always use this medicine exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
• If you have just started to use this medicine instead of, or as well as, taking corticosteroid tablets, see section 2, Patients who are already taking corticosteroid tablets.
How much Alvesco Takeda should I take each day?
Your doctor will have spoken to you about how much of your medicine you need to take each day. This will depend on your individual need.
• The recommended dose of Alvesco Takeda is 160 micrograms once daily, which leads to asthma control in the majority of patients.
• In some patients a dose reduction to 80 micrograms, once daily, may be an adequate dose for maintaining effective control of their asthma.
• An increased dosage of Alvesco Takeda may become necessary for a short period of time in patients who suffer a severe worsening of their asthma symptoms. This can be up to 640 micrograms per day, delivered as 320 micrograms twice daily but no data confirming the additional therapeutic effect after 3 months with these higher doses are available.
If necessary, your doctor may also prescribe corticosteroid tablets and/or, in the case of an infection, an antibiotic.
- Your doctor will adjust your dose to the minimum necessary to control your asthma.
- You should start to notice an improvement in your symptoms (wheezing, tight chest and coughing) within 24 hours.
When should I use my Alvesco Takeda inhaler?
In most cases, either in the morning or in the evening - as one or two puffs once a day. Follow your doctor's instructions very carefully. It is important that you take Alvesco Takeda regularly every day, even if you feel better.
If you find that you have to use your Reliever inhaler more than 2-3 times a week, you should contact your doctor to have your medicine reviewed.
How do I use my Alvesco Takeda inhaler?
Follow the instructions below. If you have any questions, ask your doctor, nurse or pharmacist.
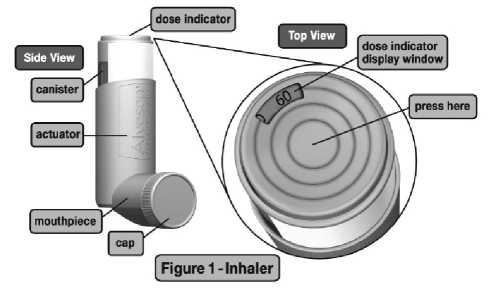
Your Alvesco Takeda inhaler is fitted with a dose indicator which shows you how much of your medicine is left during use. The dose indicator display will move every tenth time you take a puff. The dose indicator display window will turn red when there are only 20 puffs remaining. This means that you need to replace your inhaler soon. The canister should be discarded when the dose indicator display window shows zero.
While your inhaler is fitted with a dose indicator to help determine the approximate number of puffs remaining, you should keep track of the number of puffs used from each canister of Alvesco Takeda (see Step 10 below).
First use
On the first use of your Alvesco Takeda inhaler, as with all aerosol medicine, you should “test spray” the inhaler. To do this, remove the plastic cap and check the dose indicator on top of the inhaler to ensure that the dose indicator display window pointer is before the “60” (for the 60 puff pack) or “120” (for the 120 puff pack) inhalation mark before the first use. The “test spray” should also be done if the inhaler has not been used for a week or more. Spray 3 times into the air, away from the face by pressing firmly onto the centre (not ‘off centre') of the dose indicator button, indicated by the middle of the three concentric rings on the button (see Figure 1). Be sure the canister is firmly seated in the actuator before each use and that you press the inhaler slowly and firmly until it moves no further in the actuator for each spray.
You do not need to shake your Alvesco Takeda inhaler before using it. The medicine is already in a very fine solution, mixed to ensure you receive the correct dose with each puff.
Check the dose indicator before the first use after the “test spray.” Check that the dose indicator display window shows that the correct number of inhalations is left in the inhaler. If you have been prescribed a 60 puff pack, the dose indicator display window should show that there are 60 inhalations left in the inhaler. If you have been prescribed a 120 puff pack, the dose indicator display window should show that there are 120 inhalations left in the inhaler. If this is not the case, please return it to the pharmacy.

Instructions for use
It is important that a doctor, nurse or pharmacist shows you first how to use your Alvesco Takeda inhaler properly. A good technique will make sure you are receiving the correct amount into your lungs. Please use the instructions in this leaflet as a reminder.
You may wish to practice in front of the mirror for the first couple of times until you are confident that you are using your Alvesco Takeda inhaler properly. Make sure that none of your medicine is escaping from the top or sides of your mouth.
During inhalation, you can either be sitting down or standing up.
Follow these instructions carefully and use the pictures to guide you.

I.Remove the mouthpiece cover and check the mouthpiece, both the inside and the outside, to make sure that it is both clean and dry.

2. Hold the inhaler upside down (base of the canister at the top) with your forefinger on the base of the canister and your thumb under the mouthpiece.
3. Breathe out as far as is comfortable. Do not breathe out through the inhaler.

4. Place the mouthpiece in your mouth and close your lips firmly around it.
5. Just after starting to breathe in through your mouth, press down with your forefinger on the top of the inhaler to release a puff of the medicine while you are still breathing in slowly and deeply. Please take care that the puff of medicine does not escape through the top, bottom or sides of your mouth.

6.Hold your breath, take the inhaler from your mouth and remove your finger from the top of the inhaler. Continue holding your breath for about ten seconds or as long as is comfortable. Breathe out slowly through your mouth. Do not breathe out through the inhaler.
It is important that you do not rush steps 3 to 6.
|
fj | |||
|
. I |
O' | ||
|
Mb | |||
|
- iT\ | |||
|
J 4 |
mill 4 | ||
|
_/ |
7. If you have been instructed to take another puff, wait about half a minute and repeat steps 3 to 6.
8. After use, always replace the mouthpiece cover to keep out dust. Replace firmly and snap into position.
9.For hygiene reasons
- please clean the mouthpiece weekly with a dry tissue, both inside and out.
- using a dry, folded tissue, wipe over the front of the small hole where the medicine comes out.
- do not use water or any other liquids.
A correct technique will ensure the right amount of Alvesco Takeda is getting into your lungs every time you use your inhaler. Your doctor will check your inhalation technique regularly to ensure that your treatment can have the very best effect.
10 DISCARD THE ALVESCO TAKEDA INHALER WHEN THE DOSE INDICATOR DISPLAY WINDOW SHOWS ZERO. The correct amount of medicine in each inhalation cannot be assured after this point. Note: If the inhaler is dropped, do not rely on the dose indicator. It is recommended to keep track of the number of inhalations taken from your inhaler based on your records.
If you begin to feel wheezy or tightness in the chest after using your Alvesco Takeda inhaler:
• do not take any more puffs.
• use your reliever inhaler to help your breathing.
• contact your doctor immediately.
If you find it difficult to use the inhaler, your doctor may recommend the use of a spacer. The spacer that fits the Alvesco Takeda inhaler is called AeroChamber Plus™. If you use the AeroChamber Plus™ device, please follow the instructions provided with it. Your doctor or pharmacist will be able to advise you about the device.
If you use more Alvesco Takeda than you should
It is important that you take your dose as advised by your doctor. You should not increase or decrease your dose without seeking medical advice.
There is no specific treatment necessary if you have used too much Alvesco Takeda but you should inform your doctor. If high doses are used over long periods, a certain degree of reduction in adrenal function cannot be ruled out and control of the adrenal function may be necessary.
If you forget to use Alvesco Takeda
If you have forgotten to use your Alvesco Takeda, just take your usual dose when it is next due. Do not take a double number of puffs to make up for the forgotten dose.
If you stop using Alvesco Takeda
Even if you feel better, you should not stop using your Alvesco Takeda inhaler.
If you do stop using this medicine, you must tell your doctor immediately.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Alvesco Takeda can cause side effects, although not everybody gets them.
If you notice any of the following serious side effects, stop using this medicine and talk to your doctor straight away:
• severe allergic reactions such as swelling of lips, tongue and throat (may affect up to 1 in 1,000 patients treated)
• allergic reactions: skin rashes, redness, itching or weals like nettle rash and hives (may affect up to 1 in 100 patients treated)
• cough, or wheezing, which gets worse soon after taking an inhalation (may affect up to
1 in 100 patients treated)
The other side effects seen with Alvesco Takeda are usually mild. In most cases you can continue with your treatment. The side effects you may experience are:
Uncommon side effects (may affect up to 1 in 100 patients treated):
• hoarseness
• burning, inflammation, irritation of mouth or throat
• oral thrush (oral fungal infection)
• headache
• bad taste
• dryness of mouth or throat
• nausea or vomiting
Rare side effects (may affect up to 1 in 1,000 patients treated):
• sensation of heartbeat (palpitations)
• discomfort or pain in the abdomen
• high blood pressure
Frequency not known, but may also occur:
• Sleeping problems, depression or feeling worried, restless, nervous, over-excited or irritable. These effects are more likely to occur in children
Alvesco Takeda may affect the normal production of corticosteroids in your body. This is usually seen in patients taking high doses over a long period of time. These effects may include:
- reduced rate of growth in adolescents
- a thinning of the bones
- possible clouding of the lens of the eye (cataracts) causing blurred vision
- loss of vision caused by abnormally high pressure in the eye (glaucoma)
- moon-shaped face, weight gain in the upper body and thinning arms and legs (Cushingoid features or Cushing syndrome).
Adolescents who are receiving treatment for a long period of time should have their height checked regularly by their doctor. If your growth rate is slowed, your doctor will adjust your treatment if possible to the lowest dose at which effective control of asthma is maintained.
Corticosteroid tablets can lead to more side effects than a corticosteroid inhaler such as Alvesco Takeda. If you have been taking steroid tablets before or during the use of Alvesco Takeda, the risk of side effects from the tablets may continue for a period of time. Regular check-ups with your doctor will ensure that you are taking the right dose of Alvesco Takeda for you. Regular check-ups will also identify any side effects early on and reduce the chances of them worsening.
Please remember:
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE ALVESCO TAKEDA
Keep out of the sight and reach of children.
Do not use your inhaler after the expiry date which is stated on the label and the carton after EXP. The expiry date refers to the last day of that month.
The container contains a pressurised liquid.
Do not store above 50°C.
The container should not be punctured, broken or burned even if it seems empty.
As with most inhaled medicines in pressurised containers, the healing effect of this medicinal product may become smaller when the container is cold. However, Alvesco Takeda delivers the same level of dose from minus 10°C to plus 40°C.
If your doctor decides to stop treatment or if the inhaler is empty, return it to your pharmacist for safe disposal. This is important as traces of medicine could remain in the container even if you have the impression that it might be empty.
6. Contents of the pack and other information
What Alvesco Takeda contains
- The active ingredient is ciclesonide. Each actuation releases a puff (dose delivered through the mouthpiece) that contains either 40, 80 or 160 micrograms of ciclesonide.
- The other ingredients are anhydrous ethanol and propellant (HFA-134a, Norflurane).
What Alvesco Takeda looks like and contents of the pack
Alvesco Takeda consists of a clear and colourless liquid in a pressurised aluminium container which delivers through a mouthpiece an accurately measured dose of ciclesonide in the form of a spray. The inhaler is fitted with a dose indicator to show you how much of your medicine is left in the canister.
Pack sizes:
Inhaler with 60 accurately measured puffs.
Inhaler with 120 accurately measured puffs.
Each strength of inhaler contains enough for 60 or 120 puffs. Depending on the number of puffs per day your physician has recommended you to use:
• the inhaler with 60 puffs has enough medication for one to two months.
• the inhaler with 120 puffs contains enough medication for two to four months.
Not all pack sizes may be marketed in all countries.
Marketing Authorisation Holder
Takeda GmbH Byk-Gulden-Str. 2 D-78467 Konstanz Germany
Manufacturer
Takeda GmbH Byk-Gulden-Str. 2 D-78467 Konstanz Germany
This medicinal product is authorised in the Member States of the EEA under the following names:
Netherlands
Alvesco Takeda 160<80><40> Inhalator United Kingdom
Alvesco Takeda160<80><40> Inhaler This leaflet was last revised in 04/2015