Amorolfine 5% W/V Medicated Nail Lacquer
Out of date information, search anotherPACKAGE LEAFLET: INFORMATION FOR THE USER
Amorolfine 5% w/v Medicated Nail Lacquer Amorolfine
Read all of this leaflet carefully because it contains important information for you.
This medicine is available without prescription. However, you still need to use Amorolfine 5% w/v Medicated Nail Lacquer carefully to get the best results from it.
• Keep this leaflet. You may need to read it again.
• Ask your pharmacist if you need more information or advice.
• You must contact a doctor if your symptoms worsen or do not improve within 3 months.
• If you get any side effects, talk to your doctor or pharmacist/doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
In this leaflet:
1. What Amorolfine 5% w/v Medicated Nail Lacquer is and what it is used for
2. Before you use Amorolfine 5% w/v Medicated Nail Lacquer
3. How to use Amorolfine 5% w/v Medicated Nail Lacquer
4. Possible side effects
5. How to store Amorolfine 5% w/v Medicated Nail Lacquer
6. Further information
1. What Amorolfine 5% w/v Medicated Nail Lacquer is and what it is used for
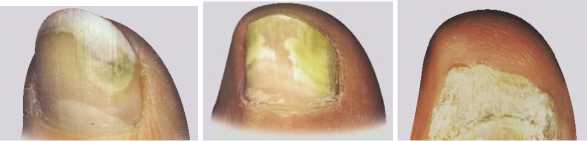
• Amorolfine 5% w/v Medicated Nail Lacquer is used to treat fungal infections affecting up to 2 nails and affecting the upper half or sides of the nail (as shown in the first picture below). If the infection appears to be more like pictures 2 or 3, you should consult your doctor.
• The active substance in Amorolfine 5% w/v Medicated Nail Lacquer is amorolfine (as the hydrochloride) which belongs to a group of medicines known as antifungals.
• Amorolfine 5% w/v Medicated Nail Lacquer kills a wide variety of fungi that can cause nail infections. A fungal nail infection is likely to result in discoloured (white, yellow or brown), thick or brittle nails, although their appearance can vary considerably as the following pictures show:
2. Before you use Amorolfine 5% w/v Medicated Nail Lacquer
Do not use Amorolfine 5% w/v Medicated Nail Lacquer if you are:
• allergic (hypersensitive) to amorolfine or any of the other ingredients of Amorolfine 5% w/v Medicated Nail Lacquer (see section 6 for other ingredients).
Take special care with Amorolfine 5% w/v Medicated Nail Lacquer:
• if you suffer from diabetes.
• if you are being treated because you have a weak immune system.
• if you have poor circulation in your hands and feet.
• if your nail is severely damaged or infected.
• if you get Amorolfine 5% w/v Medicated Nail Lacquer in your eyes or ears wash it out with water immediately and contact your doctor, pharmacist or nearest hospital straight away.
• avoid the lacquer coming into contact with mucous membranes (e.g. mouth and nostrils). Do not breathe it in.
Children and adolescents
Amorolfine 5% w/v Medicated Nail Lacquer is not recommended for use in children due to a lack of data on safety or efficacy.
Using other medicines
You can use the nail lacquer whilst you are taking other medicines.
Using other nail products
Nail varnish or artificial nails should not be used while using Amorolfine 5% w/v Medicated Nail Lacquer.
Handling of organic solvents
When organic solvents are used impermeable gloves shall be used otherwise Amorolfine 5% w/v Medicated Nail Lacquer will be removed.
Pregnancy and breast-feeding
Tell your doctor if you are pregnant, planning to become pregnant or are breast-feeding. Your doctor will then decide whether you should use Amorolfine 5% w/v Medicated Nail Lacquer.
Ask your doctor or pharmacist for advice before taking any medicine.
Always use Amorolfine 5% w/v Medicated Nail Lacquer exactly as your doctor or pharmacist has told you. You should check with your doctor or pharmacist if you are not sure.
Adults and the Elderly
Before Commencing Treatment:
In the diagram below, shade the area affected by the fungal nail infection. This will help you in remembering how the nail looked originally when your treatment is reviewed. Every three months, shade the area now affected until the infected nail has completely grown out. If more than one nail is affected, select the worst affected nail for this exercise. Take this leaflet to the pharmacist or podiatrist when treatment is being reviewed to inform them of treatment progress to date.
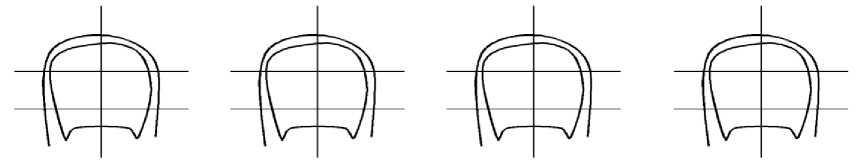
before treatment
Three Months
Six Months
Nine Months
Instructions for use:
• Treat your infected nails as described below. NAILS SHOULD BE TREATED ONCE A WEEK.
• Once weekly application should continue for 6 months for finger nails and 9 to 12 months for toe nails.
• Nails grow slowly so it may take 2 or 3 months before you start to see an improvement.
• It is important to carry on using the nail lacquer until the infection has cleared and healthy nails have grown back.
• The following steps should be followed carefully for each affected nail:
Step 1: File the nail
Before the first application, file down the infected areas of nail, including the nail surface, as much as possible using the nail file provided.
CAUTION: Do not use nail files used for infected nails on healthy
|
nails, otherwise you may spread the infection. To prevent the spread of infection take care that no one else uses the files from your kit. | ||
|
Step 2: Clean the nail Use one of the swabs provided (or nail varnish remover) to clean the nail surface. Repeat steps 1 and 2 for each affected nail. |
|
Step 3: Apply the lacquer Dip one of the re-usable applicators into the bottle of nail lacquer. The lacquer must not be wiped off the edge of the bottle before it is applied Apply the lacquer evenly over the entire surface of the nail. Repeat this step for each affected nail. Let the treated nail(s) dry for approximately 3 minutes. |
^—j 1 | |
|
Step 4: Clean the applicator The applicators provided are re-usable. However, it is important to clean them thoroughly after completing each treatment procedure, using the same swab you used for nail cleansing. Avoid touching newly treated nails with the swab. Close the nail lacquer bottle tightly. Dispose of the swab carefully as it is inflammable. |
• Before using the nail lacquer again, first remove the old lacquer from your nails using a swab, then file down the nails again if necessary.
• Re-apply the lacquer as described above.
• When dry the nail lacquer is unaffected by soap and water, so you can wash your hands and feet as normal. If you need to use chemicals such as paint thinners or white spirit, rubber or other impermeable (waterproof) gloves should be worn to protect the lacquer on your fingernails.
• It is important to carry on using the nail lacquer until the infection has cleared and healthy nails have grown back. This usually takes 6 months for fingernails and 9 to 12 months for toenails. You will see a healthy nail growing as the diseased nail grows out.

Before treatment After treatment
If you accidentally swallow Amorolfine 5% w/v Medicated Nail Lacquer
If you, or anyone else, accidentally swallows the lacquer contact your doctor, pharmacist or nearest hospital straight away.
If you forget to use Amorolfine 5% w/v Medicated Nail Lacquer
Do not worry if you forget to use the lacquer at the right time. When you remember, start using the product again, in the same way as before.
If you stop using Amorolfine 5% w/v Medicated Nail Lacquer
Do not stop using Amorolfine 5% w/v Medicated Nail Lacquer before your doctor tells you to or your infection could come back. If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Amorolfine 5% w/v Medicated Nail Lacquer can cause side effects, although not everybody gets them.
Rare side effects (occurring in less than 1 in 1000 people)
Your nail may become discoloured or it may become loose or start to separate from the nail bed.
Very rare side effects (occurring in less than 1 in 10,000 people)
A burning sensation or allergic skin reaction (contact dermatitis) may occur in the area around the nail.
Unknown frequency (frequency cannot be estimated from available data)
Redness of the skin (erythema), pruritus (itching), skin rash (urticaria), blister.
Reporting of side effects
If you get side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard By reporting side effects you can help provide more information on the safety of this medicine.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
5. How to store Amorolfine 5% w/v Medicated Nail Lacquer
• Keep out of the reach and sight of children.
• Do not use Amorolfine 5% w/v Medicated Nail Lacquer after the expiry date which is stated on the pack. The expiry date refers to the last day of that month.
• Store below 30°C. Protect from heat. Keep the bottle tightly closed and upright.
This product is flammable! Keep the solution away from fire and flames!
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Further information
What Amorolfine 5% w/v Medicated Nail Lacquer contains:
Amorolfine 5% w/v Medicated Nail Lacquer contains 50 mg/ml of the active substance amorolfine (equivalent to 55.74 mg /ml amorolfine hydrochloride). The other ingredients are Eudragit RL 100 (ammonio methacrylate copolymer A), triacetin, butyl acetate, ethyl acetate and ethanol (anhydrous).
What Amorolfine 5% w/v Medicated Nail Lacquer looks like and contents of the pack
Amorolfine 5% w/v Medicated Nail Lacquer is a clear, colourless to pale yellow solution. It is available in
3 ml and 5 ml packs; 1 bottle packed with cleansing swabs, spatulas and nail files.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing authorisation holder:
TEVA UK Limited, Eastbourne, BN22 9AG
Manufacturer:
Chanelle Medical, Loughrea, Co. Galway, Ireland This leaflet was last approved in: 10/2013
PL 00289/1683