Anbesol Teething Gel

ANBESOL® TEETHING GEL
Lidocaine Hydrochloride, Chlorocresol and Cetylpyridinium Chloride
PATIENT INFORMATION LEAFLET
Read all of this leaflet carefully before using this medicine as it contains important information for you.
• Keep this leaflet You may wish to read it again.
• Ask your pharmacist if you need more information or advice.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What Anbesol Teething Gel is and what it is used for
2. What you need to know before you use Anbesol Teething Gel
3. Howto use Anbesol Teething Gel
4. Possible side effects
5. Howto store Anbesol Teething Gel
6. Contents of pack and other information
11. What Anbesol Teething Gel is and what it is used for
lAnbesol Teething Gel contains 1.0%w/w of lidocaine 'hydrochloride, 0.1%w/w of chlorocresol and 0.02%wA/v of cetylpyridinium chloride.
Lidocaine hydrochloride is a local anaesthetic which works by 'stopping the sensation of pain. Chlorocresol and cetylpyridinium chloride kill bacteria.
Anbesol Teething Gel is used for the temporary relief of pain caused by teething, recurrent mouth ulcers and denture irritation
2. What you need to know before you use Anbesol Teething Gel
Do not use Anbesol Teething Gel:
• If you are allergic to lidocaine hydrochloride, chlorocresol, cetylpyridinium chloride, other anaesthetics similar to | lidocaine or any of the other ingredients in this medicine I (listed in Section 6).
• You have porphyria (a disease which causes stomach pain, | constipation, changes in the colour of urine, skin rashes and disturbed behaviour).
Warnings and precautions
Talk to your doctor or pharmacist before using Anbesol Teething Gel
• If you have open wounds or damaged areas in the lining of your mouth as this may result in too much of the active ingredients being absorbed.
• If symptoms continue for more than 7 days or recur frequently, speak to your pharmacist, doctor or dentist.
Do not use more Anbesol Teething Gel than recommended (as listed in Section 3) as this may result in the active ingredients being absorbed into the body causing serious side effects, such as convulsions (fits).
Other medicines and Anbesol Teething Gel
Teii your doctor or pharmacist if you are taking, have recently taken or might take any of the following medicines as they may interfere with Anbesol Teething Gel:
• Other anaesthetics or medicines similar to lidocaine for example antiarrhythmic drugs such as mexiletine which are used to control abnormal heartbeats
• Erythromycin a type of antibiotic used to treat bacterial infections
• Itraconazole a type of antifungal medicine used to treat fungal infections
• Antiarrhythmic drugs such as amiodarone which are used to control abnormal heartbeats
• Cimetidine used to treat stomach ulcers or heartburn
• Beta-blockers used to treat heart conditions, high blood pressure or migraine
• Calcium chloride used for the treatment of hypocalcaemia or as a food additive
• Codeine phosphate used to treat pain, diarrhoea or relieve coughs
• Diamorphine hydrochloride a painkiller used to treat severe pain
• Paveretum used to treat moderate to severe pain and to calm you before an operation
• Quinine hydrochloride used to treat malaria or leg cramps
• Methylceiiulose used to treat constipation
• Non-ionic surfactants such as Cetomacrogrol 1000 and polysorbate 80 which are used in medicines, foods and cosmetics
L
[Pregnancy, fertility and breast-feeding
If you are pregnant, breast-feeding, think you may be pregnant lor are planning to have a baby, ask your doctor or pharmacist Ifor advice before using this medicine, if you are breast-feeding small amounts of the active ingredient llidocaine will pass into your breast milk but in such small Iquantities that it should not harm your baby if this medicine is used as directed.
(Driving and using machines
Anbesol Teething Gel is unlikely to affect your ability to drive or use machines.
Important information about some of the ingredients
This medicinal product contains chlorocresol and the azo dye Ponceau 4R (E124) which may cause allergic reactions in sensitive individuals.
This medicinal product contains 66.605% ethanol (alcohol), i.e. iup to 0.754 ml per dose, equivalent to 15.1 ml beer, 6.3 ml wine |per dose which may be harmful for those suffering from alcoholism. To be taken into account in pregnant or breast-feeding women, children and high risk groups such as patients with liver disease, or epilepsy.
3. Howto use Anbesol Teething Gel |
Adults, the elderly and children:
• Apply a small amount of gel to a clean fingertip and spread , gently onto the sore area of the gum.
• Two applications straight away will normally be enough for temporary pain relief.
i* Do not apply more frequently than every 3 hours.
Babies only:
• Apply a small amount of gel to a clean fingertip and spread I gently onto the sore area of the gum.
• Do not apply more frequently than every 3 hours.
If you use more Anbesol Teething Gel than you should:
If anyone used more Anbesol Teething Gel than they should, or if large quantities of the gel are accidentally swallowed, contact your doctor or your nearest Accident and Emergency department, taking this leaflet and pack with you.
4. Possible side effects
Like all medicines, this medicine may cause side effects, although not everybody gets them.
I* Allergic reactions (eg difficulty breathing, rash or itch).
• Some people have reported suffering from ulcers. —|
• Dermatitis (inflammation of the skin that can cause red, itchy and scaly skin).
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist.
This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
| 5. Howto store Anbesol Teething Gel
• Keep out of the sight and reach of children.
• Do not store above 25°C.
• Do not use after the expiry date which is stated on the carton and tube after EXP. The expiry date refers to the last day of that month.
• Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
| 6. Contents of the pack and other information What Anbesol Teething Gel contains
Anbesol Teething Gel contains the active ingredients; lidocaine hydrochloride 1.0%w/w, chlorocresol 0.1%w/wand cetylpyridinium chloride 0.02%w/w.
It also contains alcohol, clove oil, glycerol, hydroxypropyl cellulose, sodium saccharin, Ponceau 4R (E124) and purified water.
What Anbesol Teething Gel looks like and contents of the pack
Anbesol Teething Gel is a soft pink clear gel for oromucosal use supplied in a tube containing 10g.
Marketing Authorisation Holder: Alliance Pharmaceuticals Limited, Avonbridge House, Bath Road, Chippenham, Wiltshire, SN15 2BB, UK.
Manufactured by: Wrafton Laboratories Limited, Wrafton, Braunton, EX33 2DL, UK.
This leaflet was last revised in March 2015 Anbesol is a registered trademark in the UK of Alliance Pharmaceuticals Limited.
Alliance and associated devices are registered trademarks of Alliance Pharmaceuticals Limited.
XXXXXI
|
Product Title: |
Anbesol Teething Gel | ||
|
Date: |
24-03-15 |
Product Size: |
140mm x 150mm |
|
Label Number: |
14-292 Version 2 |
Colours Used: |
BLACK, |
|
Fonts Used: |
Arial, Arial Bold | ||
|
Font size (min): |
6pt |
Artwork by: | |
Keylines, Guides, Dimensions and Cutter guide are NOT to be printed.
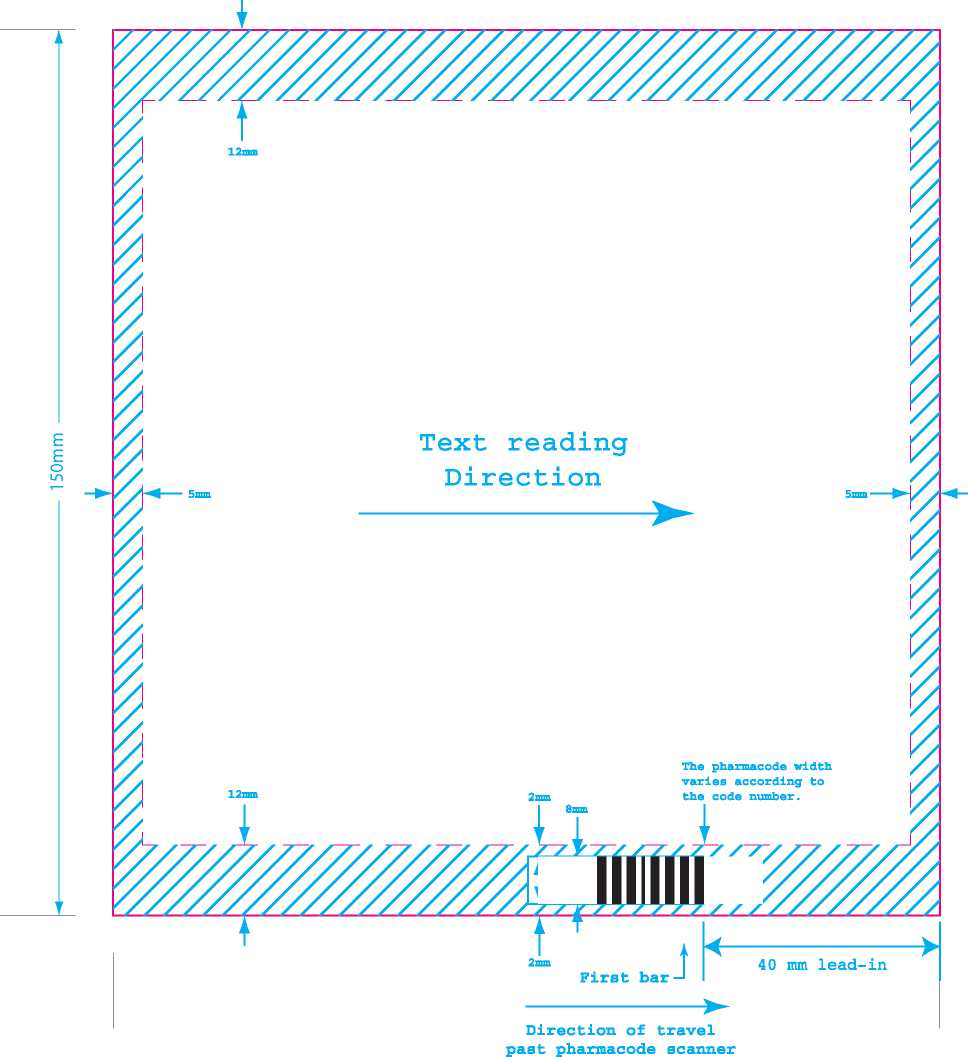
Direction of travel past pharmacode scanner
-First bar
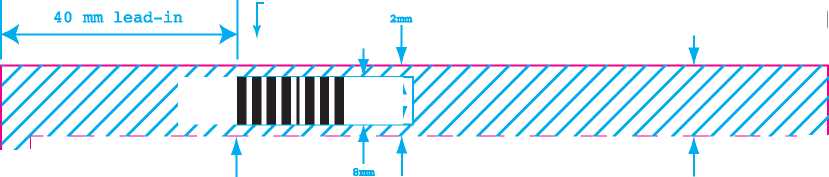
The pharmacode width varies according to the code number.
150mm
I
I
'yr
$
$
Text reading Direction
140mm
I Artwork in this I area only

Text + Print free
Pharmacode
Positioning
Pharmacode Dimensions (NOT TO SCALE)
i