Anectine 50 Mg/Ml Injection
1 NAME OF THE MEDICINAL PRODUCT
Anectine 50 mg/ml Injection
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Suxamethonium chloride Injection BP 100 mg in 2 ml.
For a full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Solution for injection.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Used in anaesthesia as a muscle relaxant to facilitate endotracheal intubation, mechanical ventilation and a wide range of surgical and obstetric procedures.
It is also used to reduce the intensity of muscular contractions associated with pharmacologically or electrically-induced convulsions.
4.2 Posology and method of administration
Usually by bolus intravenous injection.
Adults: The dose is dependent on body weight, the degree of muscular relaxation required, the route of administration, and the response of individual patients.
To achieve endotracheal intubation Anectine is usually administered intravenously in a dose of 1 mg/kg. This dose will usually produce muscular relaxation in about 30 to 60 seconds and has a duration of action of about 2 to 6 minutes. Larger doses will produce more prolonged muscular relaxation, but doubling the dose does not necessarily double the duration of relaxation. Supplementary doses of Anectine of 50% to 100% of the initial dose administered at 5 to 10 minute intervals will maintain muscle relaxation during short surgical procedures performed under general anaesthesia.
For prolonged surgical procedures Anectine may be given by intravenous infusion as a 0.1% to 0.2% solution, diluted in 5% glucose solution or sterile isotonic saline solution, at a rate of 2.5 to 4 mg per minute. The infusion rate should be adjusted according to the response of individual patients.
The total dose of Anectine given by repeated intravenous injection or continuous infusion should not exceed 500 mg per hour.
Children: Infants and young children are more resistant to Anectine compared with adults.
The recommended intravenous dose of Anectine for neonates and infants is 2 mg/kg. A dose of 1 mg/kg in older children is recommended.
When Anectine is given as intravenous infusion in children, the dosage is as for adults with a proportionately lower initial infusion rate based on body weight.
Anectine may be given intramuscularly to infants at doses up to 4 to 5 mg/kg and in older children up to 4 mg/kg. These doses produce muscular relaxation within about 3 minutes. A total dose of 150 mg should not be exceeded.
Use in the elderly: Dosage requirements of Anectine in the elderly are comparable to those for younger adults.
The elderly may be more susceptible to cardiac arrhythmias, especially if digitalis-like drugs are also being taken (see section 4.4).
Use in renal impairment: A normal single dose of suxamethonium injection may be administered to patients with renal insufficiency in the absence of hyperkalaemia. Multiples or larger doses may cause clinically significant rises in serum potassium and should not be used (see section 4.3 and 4.4).
Use in hepatic impairment: Termination of the action of suxamethonium is dependent on plasma cholinesterase, which is synthesised in the liver. Although plasma cholinesterase levels often fall in patients with liver disease, with the exception of severe hepatic failure, levels are seldom low enough to significantly prolong suxamethonium-induced apnoea (see section 4.4).
Use in patients with reduced plasma cholinesterase: Patients with reduced plasma cholinesterase activity may experience prolonged and intensified neuromuscular blockade following administration of suxamethonium. In these patients it may be advisable to administer reduced doses of suxamethonium injection (see section 4.3, 4.4 and 4.5).
Monitoring advice: Monitoring of neuromuscular function is recommended during infusion of suxamethonium injection or if suxamethonium injection is to be administered in relatively large cumulative doses over a relatively short period of time in order to individualise dosage requirements (see section 4.4).
Instructions to open the ampoule: See section 6.6 Special precautions for disposal and other handling for further information.
4.3 Contraindications
Anectine has no effect on the level of consciousness and should not be administered to a patient who is not fully anaesthetised.
Hypersensitivity to suxamethonium may exist in rare instances, and Anectine should not be administered to patients known to be hypersensitive to the drug.
As suxamethonium can act as a trigger of sustained myofibrillar contraction in susceptible individuals, Anectine is contraindicated in patients with a personal or family history of malignant hyperthermia. If this condition occurs unexpectedly, all anaesthetic agents known to be associated with its development (including Anectine) must be immediately discontinued, and full supportive measures must be immediately instituted. Intravenous dantrolene sodium is the primary specific therapeutic drug and is recommended as soon as possible after the diagnosis is made.
Anectine is contraindicated in patients known to have an inherited atypical plasma cholinesterase activity.
An acute transient rise in serum potassium often occurs following the administration of Anectine in normal individuals; the magnitude of this rise is of the order of 0.5 mmol/litre. In certain pathological states or conditions this increase in serum potassium following Anectine administration may be excessive and cause serious cardiac arrhythmias and cardiac arrest. For this reason the use of Anectine is contraindicated in:
• patients recovering from major trauma or severe burns; the period of greatest risk of hyperkalaemia is from about 5 to 70 days after the injury and may be further prolonged if there is delayed healing due to persistent infection.
• patients with neurological deficits involving acute major muscle wasting (upper and/or lower motor neurone lesions); the potential for potassium release occurs within the first 6 months after the acute onset of the neurological deficit and correlates with the degree and extent of muscle paralysis. Patients who have been immobilised for prolonged periods of time may be at similar risk.
• patients with pre-existing hyperkalaemia. In the absence of hyperkalaemia and neuropathy, renal failure is not a contra-indication to the administration of a normal single dose of Anectine Injection, but multiple or large doses may cause clinically significant rises in serum potassium and should not be used.
Suxamethonium causes a significant transient rise in intra-ocular pressure, and should therefore not be used in the presence of open eye injuries or where an increase in intra-ocular pressure is undesirable unless the potential benefit of its use outweighs the potential risk to the eye.
Anectine should be avoided in patients with a personal or family history of congenital myotonic diseases such as myotonia congenita and dystrophia myotonica since its administration may on occasion be associated with severe myotonic spasms and rigidity.
Anectine should not be used in patients with skeletal muscle myopathies e.g. Duchenne muscular dystrophy since its administration may be associated with malignant hyperthermia, ventricular dysrhythmias and cardiac arrest secondary to acute rhabdomyolysis with hyperkalaemia.
4.4 Special warnings and precautions for use
Anectine paralyses the respiratory muscles as well as other skeletal muscles but has no effect on consciousness.
Anectine should be administered only by or under close supervision of an anaesthetist familiar with its action, characteristics and hazards, who is skilled in the management of artificial respiration and only where there are adequate facilities for immediate endotracheal intubation with administration of oxygen by intermittent positive pressure ventilation.
High rates of cross-sensitivity (greater than 50%) between neuromuscular blocking agents have been reported. Therefore, where possible, before administering suxamethonium, hypersensitivity to other neuromuscular blocking agents should be excluded. Suxamethonium, should only be used when
absolutely essential in susceptible patients. Patients who experience a hypersensitivity reaction under general anaesthesia should be tested subsequently for hypersensitivity to other neuromuscular blockers.
Suxamethonium injection should not be mixed with any other drug prior to its administration.
Suxamethonium injection is acidic and should not be mixed with highly alkaline solutions, e.g. barbiturates.
During prolonged administration of Anectine, it is recommended that the patient is fully monitored with a peripheral nerve stimulator in order to avoid overdosage.
Anectine is rapidly hydrolysed by plasma cholinesterase which thereby limits the intensity and duration of the neuromuscular blockade.
Individuals with decreased plasma cholinesterase activity exhibit a prolonged response to suxamethonium . Approximately 0.05% of the population has an inherited cause of reduced cholinesterase activity.
Prolonged and intensified neuromuscular blockade following Anectine Injection may occur secondary to reduced plasma cholinesterase activity in the following states or pathological conditions:
• physiological variation as in pregnancy and the puerperium (see section 4.6)
• genetically determined abnormal plasma cholinesterase (see section 4.3)
• severe generalised tetanus, tuberculosis, other severe or chronic infections
• following severe burns (see section 4.3)
• chronic debilitating disease, malignancy, chronic anaemia and malnutrition
• end-stage hepatic failure, acute or chronic renal failure (see section 4.2)
• auto-immune diseases: myxoedema, collagen diseases
• iatrogenic: following plasma exchange, plasmapheresis, cardiopulmonary bypass, and as a result of concomitant drug therapy (see section 4.5).
If Anectine is given over a prolonged period, the characteristic depolarising neuromuscular (or Phase I) block may change to one with characteristics of a non-depolarising (or Phase II) block. Although the characteristics of a developing Phase II block resemble those of a true non-depolarising block, the former cannot always be fully or permanently reversed by anticholinesterase agents. When a Phase II block is fully established, its effects will then usually be fully reversible with standard doses of neostigmine accompanied by an anticholinergic agent.
Tachyphylaxis occurs after repeated administration of Anectine.
Muscle pains are frequently experienced after administration of suxamethonium and most commonly occur in ambulatory patients undergoing short surgical procedures under general anaesthesia. There appears to be no direct connection between the degree of visible muscle fasciculation after Anectine administration and the incidence or severity of pain. The use of small doses of non-depolarising muscle relaxants given minutes before suxamethonium administration has been advocated for the reduction of incidence and severity of suxamethonium-associated muscle pains. This technique may require the use of doses of suxamethonium in excess of 1 mg/kg to achieve satisfactory conditions for endotracheal intubation.
Caution should be exercised when using suxamethonium in children, since paediatric patients are more likely to have an undiagnosed myopathy or an unknown predisposition to malignant hyperthermia and rhabdomyolysis, which places them at increased risk of serious adverse events following suxamethonium (see section 4.3 and section 4.8).
In patients with severe sepsis, the potential for hyperkalaemia seems to be related to the severity and duration of infection.
It is inadvisable to administer Anectine to patients with advanced myasthenia gravis. Although these patients are resistant to suxamethonium they develop a state of Phase II block which can result in delayed recovery. Patients with myasthenic Eaton-Lambert syndrome are more sensitive than normal to Anectine, necessitating dosage reduction.
In healthy adults, Anectine occasionally causes a mild transient slowing of the heart rate on initial administration. Bradycardias are more commonly observed in children and on repeated administration of suxamethonium in both children and adults. Pre-treatment with intravenous atropine or glycopyrrolate significantly reduces the incidence and severity of suxamethonium-related bradycardia.
In the absence of pre-existing or evoked hyperkalaemia, ventricular arrhythmias are rarely seen following suxamethonium administration. Patients taking digitalis-like drugs are however more susceptible to such arrhythmias. The action of suxamethonium on the heart may cause changes in cardiac rhythm including cardiac arrest.
4.5 Interaction with other medicinal products and other forms of interaction
Certain drugs or chemicals are known to reduce normal plasma cholinesterase activity and may therefore prolong the neuromuscular blocking effects of Anectine. These include:
• organophosphorous insecticides and metriphonate
• ecothiopate eye drops
• trimetaphan
• specific anticholinesterase agents: neostigmine, pyridostigmine, physostigmine, edrophonium, tacrine hydrochloride
• cytotoxic compounds: cyclophosphamide, mechlorethamine, triethylene-melamine, and thiotepa
• psychiatric drugs: phenelzine, promazine and chlorpromazine
• anaesthetic agents and drugs: ketamine, morphine and morphine antagonists, pethidine, pancuronium, propanidid
• selective serotonin reuptake inhibitors (SSRI).
Other drugs with potentially deleterious effects on plasma cholinesterase activity include aprotinin, diphenhydramine, promethazine, oestrogens, oxytocin, high-dose steroids, and oral contraceptives, terbutaline and metoclopramide.
Certain drugs or substances may enhance or prolong the neuromuscular effects of Anectine by mechanisms unrelated to plasma cholinesterase activity. These include:
• magnesium salts
• lithium carbonate
• azathioprine
• quinine and chloroquine
• antibiotics such as the aminoglycosides, clindamycin and polymyxins
• antiarrhythmic drugs: quinidine, procainamide, verapamil, beta-blockers, lidocaine and procaine
• volatile inhalational anaesthetic agents: halothane, enflurane, desflurane, isoflurane, diethylether and methoxyflurane have little effect on the Phase I block of Anectine injection but will accelerate the onset and enhance the intensity of a Phase II suxamethonium-induced block.
Patients receiving digitalis-like drugs are more susceptible to the effects of suxamethonium-exacerbated hyperkalaemia.
4.6 Fertility, pregnancy and lactation
No studies of the effect of suxamethonium on female fertility or pregnancy have been performed.
Suxamethonium has no direct action on the uterus or other smooth muscle structures. In normal therapeutic doses it does not cross the placental barrier in sufficient amounts to affect the respiration of the infant.
The benefits of the use of suxamethonium as part of a rapid sequence induction for general anaesthesia normally outweigh the possible risk to the foetus.
Plasma cholinesterase levels fall during the first trimester of pregnancy to about 70 to 80% of their pre-pregnancy values; a further fall to about 60 to 70% of the pre-pregnancy levels occurs within 2 to 4 days after delivery. Plasma cholinesterase levels then increase to reach normal over the next 6 weeks. Consequently, a high proportion of pregnant and puerperal patients may exhibit mildly prolonged neuromuscular blockade following Anectine injection.
It is not known whether suxamethonium or its metabolites are excreted in human milk.
4.7 Effects on ability to drive and use machines
This precaution is not relevant to the use of suxamethonium injection. Suxamethonium will always be used in combination with a general anaesthetic and therefore the usual precautions relating to performance of tasks following general anaesthesia apply.
4.8 Undesirable effects
Adverse reactions are listed below by system organ class and frequency. Estimated frequencies were determined from published data. Frequencies are defined as follows: very common (>1/10); common (>1/100 to <1/10), uncommon (>1/1,000 to <1/100); rare (>1/10,000 to <1/1,000); very rare (<1/10,000).
Immune system disorders
Very rare Anaphylactic reactions.
Eye disorders
Common Increased intraocular pressure.
Cardiac disorders
Common Bradycardia, tachycardia.
Rare Arrhythmias (including ventricular arrhythmias),
cardiac arrest.
There are case reports of hyperkalaemia-related cardiac arrests following the administration of suxamethonium to patients with congenital cerebral palsy, tetanus, Duchenne muscular dystrophy, and closed head injury. Such events have also been reported rarely in children with hitherto undiagnosed muscular disorders.
Vascular disorders
Common Skin flushing.
Hypertension and hypotension have also been reported.
Respiratory, thoracic and mediastinal disorders
Rare Bronchospasm, prolonged respiratory depression!, apnoeaf.
f Individuals with decreased plasma cholinesterase activity exhibit a prolonged response to suxamethonium. Approximately 0.05% of the population has an inherited cause of reduced cholinesterase activity (please refer to section 4.4).
Gastrointestinal disorders
Very common Increased intragastric pressure.
Excessive salivation has also been reported
Skin and subcutaneous tissue disorders Common Rash.
Musculoskeletal and connective tissue disorders
Very common Muscle fasciculation, post-operative muscle pains
(please refer to section 4.4).
Common Myoglobinaemia#, myoglobinuria#.
Rare Trismus
# Rhabdomyolysis has also been reported (see section 4.3 and section 4.4).
General disorders and administration site conditions
Very rare Malignant hyperthermia (please refer to section 4.4).
Investigations
Common Transient blood potassium increase.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov .uk/yellowcard.
4.9 Overdose
Apnoea and prolonged muscle paralysis are the main serious effects of overdosage. It is essential, therefore, to maintain the airway and adequate ventilation until spontaneous respiration occurs.
The decision to use neostigmine to reverse a Phase II suxamethonium-induced block depends on the judgement of the clinician in the individual case. Valuable information in regard to this decision will be gained by monitoring neuromuscular function. If neostigmine is used its administration should be accompanied by appropriate doses of an anticholinergic agent such as atropine.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Peripherally acting muscle relaxants, choline derivatives, ATC code: M03AB01.
Short-acting depolarising neuromuscular blocking agent.
5.2 Pharmacokinetic properties
None stated.
5.3 Preclinical safety data
Genotoxicity:-
No bacterial mutation assays have been conducted.
There are some data to suggest a weak clastogenic effect in mice, but not in patients who had received suxamethonium chloride.
Carcinogenicity:-
Carcinogenicity studies have not been performed.
Embryo-foetal Development:-
Animal reproduction studies have not been conducted with suxamethonium. It is also not known whether suxamethonium can affect reproductive capacity or cause foetal harm when administered to a pregnant woman.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Water for Injections EP.
6.2 Incompatibilities
None known.
Suxamethonium injection should not be mixed with any other drug prior to its administration.
6.3 Shelf life 18 months.
6.4 Special precautions for storage
Store between 2 and 8 °C. Do not freeze. Keep in the outer carton to protect from light.
6.5 Nature and contents of container
Neutral glass. 2 ml ampoules.
6.6 Special precautions for disposal and other handling
For intravenous injection under medical direction.
Instructions to open the ampoule
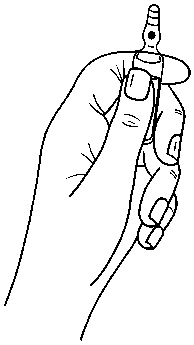
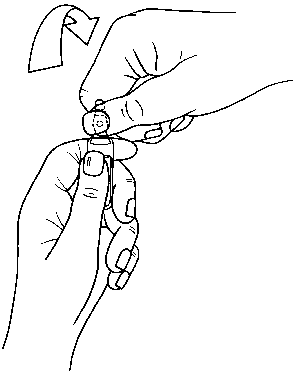
Ampoules are equipped with the OPC (One Point Cut) opening system and must be opened using the following instructions:
• hold with the hand the bottom part of the ampoule as indicated in picture 1
• put the other hand on the top of the ampoule positioning the thumb above the coloured point and press as indicated in picture 2
Picture 1
Picture 2
7 MARKETING AUTHORISATION HOLDER
The Wellcome Foundation Limited 980 Great West Road,
Brentford,
Middlesex,
TW8 9GS,
United Kingdom
trading as
GlaxoSmithKline UK Stockley Park West Uxbridge
Middlesex UB11 1BT
8 MARKETING AUTHORISATION NUMBER(S)
PL 00003/5203R
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
30 August 1985/16 May 2008
10 DATE OF REVISION OF THE TEXT
12/01/2016