Artelac 3.2 Mg/Ml Eye Drops Solution
|
Graphics&Packing Technology Section ICN POLFA RZESZOW S.A. |
ULOTKA PIL BAUSCH 1 LOMB | |||||
|
Nazwa produktu Product Name |
Artelac 3.2mg/ml Eye drops solution |
Kolor nadruku Colours | ||||
|
Black | | ||||||
|
Kraj Country (ISO) |
United Kingdom/Ireland (UK/IE) |
Nr wykrojnika Spec No |
93038 | |||
|
Opracowane przez Designed by |
Kod Wytworcy Manufacturer code |
40728PB188/7-IE/UK | ||||
|
Nr korekty Proof No |
7 |
Data 21-09-2015 Date |
Kod farmaceutyczny Pharmacode |
873 | ||
|
Kod wersji Valeant version code |
P1UKIE01 |
Inny kod Other code |
- | |||
|
Rozmiar czcionki Font size |
minimum 8 pt |
Wymiar ulotki PIL size |
120 x 270 mm | |||
|
Kroj czcionki Font used |
Helvetica Neue LT W1G 55 Roman 56 Italic 75 Bold 76 Bold Italic |
Gramatura papieru Paper weight |
- g/m2 | |||
|
Komentarze Comments (Reason for the change) | ||||||
|
- PMR_ICN-15-0626-A - Factory (Packing site): DMP Berlin | ||||||
|
Akceptacja Techniczna Technical Approval | ||||||
|
Akceptacja Dziafu ds. Rejestracji Regulatory Affairs Dept. Approval | ||||||
00
PACKAGE LEAFLET: INFORMATION FOR THE USER
Artelac® 3.2 mg/mlEye Drops, Solution
Hypromellose
The name of your medicine is Artelac 3.2 mg/ml Eye Drops, Solution and it is called Artelac in this leaflet.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Always use this medicine exactly as described in this leaflet or as your doctor or pharmacist have told you.
- Keep this leaflet. You may need to read it again.
- Ask your pharmacist if you need more information or advice.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
- You must talk to a doctor if you do not feel better or if you feel worse.
What is in this leaflet
1. What Artelac is and what it is used for
2. What you need to know before you use Artelac
3. How to use Artelac
4. Possible side effects
5. How to store Artelac
6. Contents of the pack and other information
1. WHAT ARTELAC IS AND WHAT IT IS USED FOR
Artelac contains the active ingredient hypromellose which acts as a lubricant to treat symptoms of dry eye. It is a soothing solution that is used as an artificial tear when the eyes are lacking in tears.
Your eyes will normally produce just enough natural tears to allow them to move easily and comfortably. If your eyes do not produce enough tears they can become dry, red and painful.
Your drops keep the surface of the eye lubricated (moist) when production of tears is less than normal, and has a refreshing, cooling action. The hypromellose in these drops increases the thickness of the solution which has a moisturising effect and helps the solution to stay in contact with your eye for a longer period of time.
Artelac can be used by children, adults and the elderly.
2. WHAT YOU NEED TO KNOW BEFORE YOU USE ARTELAC
Do NOT use Artelac
• If you are allergic to hypromellose or any of the ingredients of this medicine (listed in Section 6).
Warnings and precautions
• Talk to your doctor or pharmacist before using Artelac.
• If you wear soft contact lenses - take out your lenses before using Artelac and then wait for at least 15 minutes after using these drops before putting your lenses back in.
• If your eyes continue to feel dry and sore, stop using your drops and tell your doctor.
Other medicines and Artelac
• Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Pregnancy, breast-feeding and fertility
• If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
• All eye drops can cause a short term blurring of vision when first used, so you should wait until your eyes are clear before driving or using machines.
873 CZ8
Always use your solution exactly as your doctor or pharmacist has told you to. Check with your doctor or pharmacist if you are not sure.
This medicine is only to be used as drops applied into one or both eye(s).
The recommended dose is
The usual dose is to apply 1 drop in the corner of the eye, nearest the nose, 3 to 5 times each day or as required to give enough lubrication. Ensure the dropper tip does
_ not touch any surface including the eye surface.
Dry eye is a condition that varies from person to person. Use the drops as often as you think necessary.
co Directions for use
• Wash your hands before using your drops
• Sit down in front of a mirror so that you can see what you are doing
• Wipe your eyes to clear any residual wateriness or discharge
• Unscrew the cap from the bottle and check the dropper is clean
• Pull your lower eyelid gently down, and then carefully place one drop inside the
|
Graphics&Packing Technology Section ICN POLFA RZESZOW S.A. |
ULOTKA PIL BAUSCH 1 LOMB | |||||
|
Nazwa produktu Product Name |
Artelac 3.2mg/ml Eye drops solution |
Kolor nadruku Colours | ||||
|
Black | | ||||||
|
Kraj Country (ISO) |
United Kingdom/Ireland (UK/IE) |
Nr wykrojnika Spec No |
93038 | |||
|
Opracowane przez Designed by |
Kod Wytworcy Manufacturer code |
40728PB188/7-IE/UK | ||||
|
Nr korekty Proof No |
7 |
Data 21-09-2015 Date |
Kod farmaceutyczny Pharmacode |
873 | ||
|
Kod wersji Valeant version code |
P1UKIE01 |
Inny kod Other code |
- | |||
|
Rozmiar czcionki Font size |
minimum 8 pt |
Wymiar ulotki PIL size |
120 x 270 mm | |||
|
Kroj czcionki Font used |
Helvetica Neue LT W1G 55 Roman 56 Italic 75 Bold 76 Bold Italic |
Gramatura papieru Paper weight |
- g/m2 | |||
|
Komentarze Comments (Reason for the change) | ||||||
|
- PMR_ICN-15-0626-A - Factory (Packing site): DMP Berlin | ||||||
|
Akceptacja Techniczna Technical Approval | ||||||
|
Akceptacja Dziafu ds. Rejestracji Regulatory Affairs Dept. Approval | ||||||
00
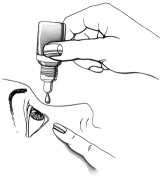
lower eyelid, in the corner nearest the nose (see picture)
• Take care not to touch the dropper with your eye or fingers
• Release the lower eyelid, and blink a few times to make sure the eye is covered by the liquid
• Repeat the procedure for your other eye
• When you have finished, put the cap back on the bottle tightly
If you use more Artelac than you should
lf you accidentally use a larger dose than recommended, this may cause some blurring of vision, which will soon pass.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
If you forget to take Artelac
Do not take a double dose to make up for a forgotten dose.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Artelac can cause side effects, although not everybody gets them. In very rare cases, some patients with severe damage to the clear layer at the front of the eye (the cornea) have developed cloudy patches on the cornea due to calcium build-up during treatment.
You may briefly have blurred vision or a slight stinging feeling after using your drops. Frequency cannot be estimated from the available data.
If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via:
United Kingdom:
Yellow Card Scheme - Website: www.mhra.gov.uk/yellowcard Ireland
HPRA Pharmacovigilance
Earlsfort Terrace, IRL - Dublin 2
Tel: +353 1 6764971; Fax: +353 1 6762517
Website: http://www.hpra.ie; E-mail: medsafety@hpra.ie
By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE ARTELAC
• Keep out of the reach and sight of children.
• Do not use this medicine after its expiry date which is stated on the label.
- The expiry date refers to the last day of that month.
- If the expiry date has passed, take the medicine back to your pharmacist.
• Do not store your drops above 25°C.
• Your eye drops are sterile until first opened. It is important to keep the drops as clean as possible during use. Do not touch the dropper tip on the eye or any other surface.
• Your drops come in bottle which should be thrown away 28 days after first opening.
• Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Artelac contains
• The active ingredient is: hypromellose 3.2 mg/ml (0.32 % w/v).
• The other ingredients are: cetrimide, disodium phosphate dodecahydrate, sodium dihydrogen phosphate dihydrate, disodium edetate, sorbitol and water for injections. The preservative in your eye drops is cetrimide, which means that Artelac can be used if your eyes are sensitive to other preservatives such as benzalkonium chloride.
What Artelac looks like and contents of the pack
These eye drops are a clear sterile solution and are supplied in plastic eye drop
bottles. Each bottle has a volume of 10 ml.
873 CZ8
Marketing Authorisation Holder
Bausch & Lomb UK Ltd., 106 London Road, Kingston-Upon-Thames, Surrey, KT2 6TN, UK
Manufacturer
Dr Gerhard Mann Chem-pharm Fabrik GmbH, 13581 Berlin, Germany
co
CD

PA555/007/001 PL 03468/0032
This leaflet was approved September 2015
®/™ are trademarks of Bausch & Lomb Incorporated or its affiliates.
© Bausch & Lomb Incorporated.
P1UKIE01 40728PB188/7-IE/UK
P1UKIE01_40728PB188_7-IE_UK_Artelac_3,2mg_ml_eye_drops_210915_07.indd 2 2015-09-21 12:32:16