Arutidor 20 Mg/Ml + 5Mg/Ml Eye Drops Solution
Out of date information, search anotherSUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Arutidor 20 mg/ml + 5mg/ml Eye Drops, Solution
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml contains 22.26 mg dorzolamide hydrochloride, equivalent to 20 mg dorzolamide and 6.83 mg timolol maleate, equivalent to 5 mg timolol. Excipients: Benzalkonium chloride 0.075 mg/ml.
For a full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Eye drops, solution
Dorzolamide/Timolol Eye Drops, Solution is a clear, colourless to light yellow, sterile eye drop solution.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Indicated for the treatment of increased intraocular pressure (IOP) in patients with open-angle glaucoma or pseudo-exfoliative glaucoma, when monotherapy with a topical beta-blocker is not sufficient.
4.2 Posology and method of administration
Posology
Adults
Dosage is one drop into the conjunctival sac of each affected eye twice daily.
Method of administration
When using nasolacrimal occlusion or closing the eyelids for 2 minutes, the systemic absorption is reduced. This may result in a decrease in systemic side effects and an increase in local activity.
If another ophthalmic agent is being administered, Dorzolamide/Timolol Eye Drops, Solution and the other agent should be applied at an interval of at least 10 minutes. Patients should be advised to wash their hands prior to administration and to avoid touching the eyes or surrounding area with the dropper tip.
Patients should also be advised that eye drops, if handled incorrectly, can become contaminated by ubiquitous bacteria, which may lead to eye infections. Serious ocular damage and subsequent loss of vision may result from using contaminated eye drops. Patients should be informed how to handle the bottle correctly.
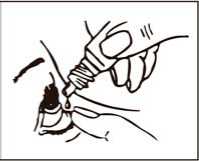
Instructions for use
1. Wash your hands and sit or stand comfortably.
2. Twist off the cap.
3. Tilt the head back.
4. Use your finger to gently pull down the lower eyelid of your affected eye.
5. Invert the bottle and place the tip of the bottle close to, but not touching your eye. DO NOT TOUCH YOUR EYE OR EYELID WITH THE DROPPER TIP.
6. Squeeze the bottle gently so that only one drop goes into your eye, then release the lower eyelid.
7. Close the eye and press a finger against the corner of the affected eye by the nose. Hold for 1 minute.
8. Repeat in your other eye if your doctor has told you to do this.
9. Put the cap back on the bottle.
Paediatric population
The efficacy of Dorzolamide / Timolol Eye Drops, Solution in children aged from birth to 18 years of age has not yet been established.
Current available data regarding safety in paediatric patients aged between 2 and 6 years are described in section 5.1 but no recommendation on a posology can be made.
4.3 Contraindications
Dorzolamide/Timolol Eye Drops, Solution is contraindicated in patients with
• hypersensitivity to one or both active substances or to any of the excipients.
• reactive airway disease, including bronchial asthma or a history of bronchial asthma, or severe chronic obstructive pulmonary disease.
• sinus bradycardia, sick sinus syndrome, sino-atrial block, second- or third-degree atrioventricular block not controlled with pace-maker, overt cardiac failure, cardiogenic shock.
• severe renal dysfunction (creatinine clearance < 30 ml/min) or hyperchloraemic acidosis
The above statements refer to the respective active substances and are not only restricted to the combination.
4.4 Special warnings and precautions for use
Like other topically applied ophthalmic agents Timolol is absorbed systemically. Due to beta-adrenergic component, Timolol, the same types of cardiovascular, pulmonary and other adverse reactions seen with systemic beta-adrenergic blocking agents may occur. Incidence of systemic ADRs after topical ophthalmic administration is lower than for systemic administration. To reduce the systemic absorption, see 4.2.
Cardiac Disorders
In patients with cardiovascular diseases (e.g. coronary heart disease, Prinzmetal's angina and cardiac failure) and hypotension, therapy with beta-blockers should be critically assessed and the therapy with other active substances should be considered. Patients with cardiovascular diseases should be watched for signs of deterioration of these diseases and of adverse reactions.
Due to its negative effect on conduction time, betablockers should only be given with caution to patients with first degree heart block.
Vascular disorders
Patients with severe peripheral circulatory disturbance/disorders (i.e. severe forms of Raynaud’s disease or Raynaud’s syndrome) should be treated with caution.
Respiratory disorders:
Following administration of ophthalmic beta-blockers, there have been reports of respiratory and cardiac reactions, including fatalities in patients with asthma due to bronchospasm and rare fatalities in patients with heart failure.
Dorzolamide/Timolol Eye Drops, Solution should be used with caution, in patients with mild/moderate chronic obstructive pulmonary disease (COPD) and only if the potential benefit outweighs the potential risk.
Hypoglycaemia/diabetes
Beta-blockers should be administered with caution in patients subject to spontaneous hypoglycaemia or to patients with labile diabetes, as beta-blockers may mask the signs and symptoms of acute hypoglycaemia.
Hepatic dysfunction
Dorzolamide/Timolol Eye Drops, Solution has not been tested in patients with hepatic dysfunction and should therefore be used with caution in such patients.
Immunology and hypersensitivity
As with other topically administered ophthalmic agents, this medicinal product may be systemically absorbed. Dorzolamide possesses a sulphonamide group that is also found in sulphonamides. Therefore, the same undesirable effects encountered in systemic sulphonamide treatment may occur with topical administration. If signs of serious reactions or hypersensitivity reactions occur, this preparation must be discontinued.
Local adverse ocular reactions, similar to those seen with dorzolamide hydrochloride eye drops, have been observed during Dorzolamide/Timolol Eye Drops, Solution treatment. If such reactions occur, discontinuation of Dorzolamide/Timolol Eye Drops, Solution therapy should be considered.
Anaphylactic reactions
During administration of beta-blockers, patients with a history of atopy or a history of severe anaphylactic reaction to various allergens may be more responsive than normal to repeated accidental, diagnostic or therapeutic exposure to such allergens. Such patients may be unresponsive to the usual adrenaline dose used in the treatment of anaphylactic reactions.
Other beta-blocking agents
The effect on intra-ocular pressure or the known effects of systemic beta-blockade may be potentiated when Dorzolamide/Timolol Eye Drops, Solution is given to the patients already receiving a systemic beta-blocking agent. The response of these patients should be closely observed. The use of two topical beta-adrenergic blocking agents is not recommended (see section 4.5).
Choroidal detachment
Choroidal detachment has been reported with administration of aqueous suppressant therapy (e.g. timolol, acetazolamide) after filtration procedures.
Surgical anaesthesia
P-blocking ophthalmological preparations may block systemic P-agonist effects e.g. of adrenaline. The anaesthesiologist should be informed when the patient is receiving Dorzolamide/Timolol Eye Drops, Solution.
Concomitant therapy
Adjuvant administration of the following medications is not recommended:
> dorzolamide and oral carbonic anhydrase inhibitors
> topical beta-receptor blockers.
Discontinuation of therapy
As with systemic beta-blockers, if discontinuation of timolol maleate eye drops becomes necessary in patients with coronary heart disease, therapy should be tapered off gradually.
Additional effects of beta-blockade
Therapy with beta-blockers can mask certain symptoms of hyperthyroidism. Abrupt discontinuation of beta-blocker therapy may precipitate exacerbation of symptoms.
Therapy with beta-blockers can aggravate symptoms of myasthenia gravis.
Additional effects of carbonic anhydrase inhibition
Therapy with oral carbonic anhydrase inhibitors has been associated with urolithiasis as a result of acid-base imbalances, particularly in patients with a prior history of kidney stones. Although no disturbances in the acid-base balance have been observed with Dorzolamide/Timolol Eye Drops, Solution, there have been rare reports of urolithiasis. As Dorzolamide/Timolol Eye Drops, Solution contains a topical carbonic anhydrase inhibitor that is systemically absorbed, there may be an increased risk of urolithiasis during Dorzolamide/Timolol Eye Drops, Solution administration in patients with a prior history of kidney stones.
Others
Treatment of patients with acute angle-closure glaucoma requires adjuvant therapeutic procedures, in addition to ocular hypotensive agents. Dorzolamide/Timolol Eye Drops, Solution has not been tested in patients with acute angle-closure glaucoma.
During use of dorzolamide, corneal oedema and irreversible corneal decompensation have been reported in patients with pre-existing chronic corneal defects and/or a history of intraocular surgery. Topical dorzolamide should be used with caution in such patients.
Ophthalmic P-blockers may induce dryness of eyes. Patients with corneal diseases should be treated with caution.
As with the use of other antiglaucoma agents, reduced response to timolol maleate eye drops has been reported in some patients after prolonged therapy. However, in clinical studies where 164 patients were monitored for at least 3 years, no significant changes in mean intraocular pressure were observed after initial stabilisation.
Use of contact lenses
Dorzolamide/Timolol Eye Drops, Solution contains benzalkonium chloride as a preservative, which may cause eye irritation. Contact lenses should be removed prior to use of the drops and should not be reinserted for at least 15 minutes postadministration. Benzalkonium chloride leads to discolouration of soft contact lenses.
Effects when misused for doping purposes
In doping tests, use of Dorzolamide/Timolol Eye Drops, Solution may lead to positive results.
Paediatric population See section 5.1.
4.5 Interaction with other medicinal products and other forms of interaction
No specific interaction studies have been performed with Dorzolamide/Timolol Eye Drops, Solution.
In clinical studies, Dorzolamide/Timolol Eye Drops, Solution was co-administered with the following systemic medications without any occurrence of interactions: ACE inhibitors, calcium channel blockers, diuretics, non-steroidal anti-inflammatory drugs including acetylsalicylic acid, and hormones (e.g. oestrogen, insulin, thyroxine).
However, effects may be potentiated - and hypotension and/or marked bradycardia may be precipitated - when timolol maleate eye drops are administered together with oral calcium channel blockers, catecholamine-depleting agents or beta-receptor agents, antiarrhythmics (including amiodarone), digitalis glycosides, parasympathomimetics, guanethidine, narcotics and monoamine oxidase (MAO) inhibitors.
Potentiated systemic beta-blockade (e.g. decreased heart rate, depression) has been reported during combined treatment with CYP2D6 inhibitors (e.g. quinidine, fluoxetine, paroxetine) and timolol.
The dorzolamide component of Dorzolamide/Timolol Eye Drops, Solution is a carbonic anhydrase inhibitor, which, although topically administered, is absorbed systemically. In clinical studies, no disturbances in the acid-base balance occurred during treatment with dorzolamide hydrochloride eye drops. However, such disturbances have been observed with administration of oral carbonic anhydrase inhibitors, leading to interactions in some cases (e.g. toxic effects during high-dose salicylate therapy). Therefore, the potential for such interactions should be considered in patients receiving Dorzolamide/Timolol Eye Drops, Solution.
Mydriasis resulting from concomitant use of ophthalmic beta-blockers and adrenaline (epinephrine) has been reported occasionally.
Upon discontinuation of clonidine, oral beta-blockers may aggravate any hypertension that may occur as a result of rebound effects.
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no adequate data for the use of Dorzolamide/Timolol Eye Drops, Solution in pregnant women. Dorzolamide/Timolol Eye Drops, Solution should not be used during pregnancy unless clearly necessary.
To reduce the systemic absorption, see 4.2.
Epidemiological studies have not revealed malformative effects but show a risk for intra uterine growth retardation when betablockers are administered by the oral route. In addition, signs and symptoms of beta-blockade (e.g. bradycardia, hypotension, respiratory distress and hypoglycaemia) have been observed in the neonate when beta-blockers have been administered until delivery. If Dorzolamide/Timolol Eye Drops, Solution is administered until delivery, the neonate should be carefully monitored during the first days of life.
There are no adequate clinical data available on pregnant women exposed to Dorzolamide. In rabbits, dorzolamide resulted in teratogenic effects at maternotoxic doses (see section 5.3).
Lactation
Beta-blockers are excreted in breast milk. However, at therapeutic doses of Dorzolamide/Timolol Eye Drops, Solution in eye drops it is not likely that sufficient amounts would be present in breast milk to produce clinical symptoms of beta-blockade in the infant. To reduce the systemic absorption, see 4.2.
It is not known whether dorzolamide passes into breast milk. A reduction in body weight gain was observed in the offspring of lactating rats given dorzolamide.
If treatment with Dorzolamide/Timolol Eye Drops, Solution is required, breastfeeding is not recommended.
4.7 Effects on ability to drive and use machines
Dorzolamide/Timolol Eye Drops, Solution has moderate influence on the ability to drive and use machines. Possible adverse reactions, such as blurred vision, may impair the ability of some patients to drive and/or use machines.
4.8 Undesirable effects
Like other topically applied ophthalmic drugs, Dorzolamide/Timolol Eye Drops, Solution is absorbed into the systemic circulation. This may cause similar undesirable effects as seen with systemic betablocking agents. Incidence of systemic ADRs after topical ophthalmic administration is lower than for systemic administration. Listed adverse reactions include reactions seen within the class of ophthalmic beta-blockers.
Additional adverse reactions have been seen with ophthalmic beta-blockers and may potentially occur with Dorzolamide/Timolol Eye Drops, Solution.
In clinical studies no adverse experiences specific to a Dorzolamide/Timolol Eye Drops, Solution have been observed; adverse experiences have been limited to those that were reported previously with dorzolamide hydrochloride and/or timolol maleate. In general, common adverse experiences were mild and did not cause discontinuation.
During clinical studies, 1,035 patients were treated with a Dorzolamide/Timolol Eye Drops, Solution. Approximately 2.4% of all patients discontinued therapy with Dorzolamide/Timolol Eye Drops, Solution because of local ocular adverse reactions. Approximately 1.2% of all patients discontinued because of local adverse reactions suggestive of allergy or hypersensitivity (such as lid inflammation and conjunctivitis).
The following adverse reactions have been reported with Dorzolamide/Timolol Eye Drops, Solutions or one of its components either during clinical trials or during postmarketing experience:
Adverse events are categorized by frequency as follows:
Very common (>=1/10)
Common (>=1/100 to <1/10)
Uncommon (>=1/1,000 to <1/100)
Rare (>=1/10,000 to <1/1,000)
Very rare (<1/10,000)
Not known (cannot be estimated from the available data)
Immune system disorders:
Timolol maleate eye drops:
Systemic allergic reactions including angioedema, urticaria, localized and generalized rash, pruritus, anaphylactic reaction.
Musculoskeletal and connective tissue disorders:
Timolol maleate eye drops:
Rare: systemic lupus erythematosus, myalgia
Nervous system disorders:
Dorzolamide hydrochloride eye drops:
Common: headache Rare: dizziness, paraesthesia Timolol maleate eye drops:
Common: headache Uncommon: dizziness, depression
Rare: insomnia, nightmares, memory loss, paraesthesia, increase in the objective and subjective symptoms of myasthenia gravis, cerebrovascular accident
Eye disorders:
Dorzolamide/Timolol Eye Drops, Solution Very common: burning and stinging
Common: conjunctival injection, blurred vision, corneal erosion, ocular
itching, lacrimation
Dorzolamide hydrochloride eye drops:
Common: eyelid inflammation, eyelid irritation Uncommon: iridocyclitis
Rare: irritation e.g. redness, pain, eyelid crusting, transient myopia (which resolved upon discontinuation of therapy), corneal oedema, ocular hypotension and choroidal detachment (after filtration surgery)
Timolol maleate eye drops:
Common: subjective and objective symptoms of ocular irritation (e.g. burning, stinging, itching, tearing, redness), including blepharitis, keratitis, reduced corneal sensitivity and dry eyes
Uncommon: visual disturbances including refractive changes (in some cases, due to discontinuation of miotic therapy)
Rare: ptosis, diplopia, choroidal detachment (following filtration surgery; see
4.4 Special warnings and special precautions for use)
Ear and labyrinth disorders: Timolol maleate eye drops:
Rare: tinnitus
Cardiac and vascular disorders:
Timolol maleate eye drops:
Uncommon: bradycardia, syncope
Rare: hypotension, chest pain, palpitations, oedema, arrhythmia, congestive heart failure, atrio-ventricular block, cardiac failure, cardiac arrest, cerebral ischaemia, claudication, Raynaud’s phenomenon, cold hands and feet
Respiratory, thoracic, and mediastinal disorders:
Dorzolamide/Timolol Eye Drops, Solution Common: sinusitis
Rare: shortness of breath, respiratory failure, rhinitis
Dorzolamide hydrochloride eye drops:
Rare: epistaxis Timolol maleate eye drops:
Uncommon: dyspnoea
Rare: bronchospasm (mainly in patients with pre-existing bronchospastic disease), cough
Gastrointestinal disorders:
Dorzolamide/Timolol Eye Drops, Solution Very common: taste perversion
Dorzolamide hydrochloride eye drops Common: nausea,
Rare: throat irritation, dry mouth Timolol maleate eye drops:
Uncommon: nausea, dyspepsia
Rare: diarrhoea, dry mouth, abdominal pain, vomiting
Skin and subcutaneous tissue disorders:
Dorzolamide/Timolol Eye Drops, Solution Rare: contact dermatitis Dorzolamide hydrochloride eye drops Rare: exanthema
Timolol maleate eye drops:
Rare: alopecia, psoriasiform rash or exacerbation of psoriasis, skin rash.
Renal and urinary disorders:
Dorzolamide/Timolol Eye Drops, Solution Uncommon: urolithiasis.
Reproductive system and breast disorders:
Timolol maleate eye drops:
Rare: Peyronie’s disease, sexual dysfunction, decreased libido.
General disorders and administration site disorders:
Dorzolamide/Timolol Eye Drops, Solution
Rare: subjective and objective symptoms of systemic allergic reactions, including angioedema, urticaria, pruritus, exanthem, anaphylaxis, bronchospasm
Dorzolamide hydrochloride eye drops:
Common: asthenia/fatigue Timolol maleate eye drops:
Uncommon: asthenia/fatigue
Metabolism and nutrition disorders:
Timolol maleate eye drops:
Hypoglycaemia
4.9 Overdose
For humans, no data are available with regard to overdosage following inadvertent or deliberate ingestion of Dorzolamide/Timolol Eye Drops, Solution.
Symptoms
There are reports of inadvertent overdosage with timolol maleate eye drops, which have led to systemic effects similar to those seen with systemic beta-receptor blockers, such as dizziness, headache, shortness of breath, bradycardia, bronchospasm and cardiac arrest. The most common objective and subjective symptoms to be expected from overdosage with dorzolamide hydrochloride are electrolyte shifts, development of acidosis and possibly effects on the CNS.
Only limited information is available with regard to human overdosage following accidental or deliberate ingestion of dorzolamide hydrochloride. Following oral ingestion, somnolence has been reported; following topical application, nausea, dizziness, headache, fatigue, abnormal dreams and dysphagia have been reported.
Treatment
Treatment should be symptomatic and supportive. Serum electrolyte levels (particularly potassium) and blood pH levels should be monitored. Studies have shown that timolol is not rapidly dialysable.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: glaucoma agents and miotics, beta-adrenoreceptor antagonists, timolol, combinations, ATC code: S01ED51
Mechanism of action
Dorzolamide/Timolol Eye Drops, Solution comprises two components: dorzolamide hydrochloride and timolol maleate. Each of these two components lowers increased intraocular pressure by reducing aqueous humor production but does so by a different mechanism of action.
Dorzolamide hydrochloride is a potent inhibitor of human carbonic anhydrase II. Inhibition of carbonic anhydrase in the ciliary processes of the eye decreases aqueous humor production, presumably by slowing down the formation of bicarbonate ions, with a subsequent reduction in sodium and fluid transport.
Timolol maleate is a non-selective beta-adrenergic receptor blocking agent. The precise mechanism of action of timolol maleate in lowering intraocular pressure, is not clearly established at this time, although a fluorescein study and tonography studies indicate that the predominant action may be related to reduced aqueous humor formation. However, in some studies a slight increase in outflow facility was also observed. The combined effect of these two agents results in additional intraocular pressure (IOP) reduction, compared to either component administered alone.
Following topical administration, Dorzolamide/Timolol Eye Drops, Solution reduces intraocular pressure, whether or not associated with glaucoma. Elevated intraocular pressure is a major risk factor in the pathogenesis of optic nerve damage and glaucomatous visual field loss. Dorzolamide/Timolol Eye Drops, Solution reduces intra-ocular pressure without the common side effects of miotics such as night-blindness, accommodative spasm and pupillary constriction.
Pharmacodynamic effects
Clinical effects:
Clinical studies of up to 15 months duration were conducted to compare the IOP-lowering effect of Dorzolamide/Timolol Eye Drops, Solution b.i.d. (dosed morning and bedtime) to individually- and concomitantly-administered 0.5% timolol and 2.0% dorzolamide in patients with glaucoma or ocular hypertension for whom concomitant therapy was considered appropriate in the trials. This included both untreated patients and patients inadequately controlled with timolol monotherapy. The majority of patients were treated with topical beta-blocker monotherapy prior to study enrolment. In an analysis of the combined studies, the IOP-lowering effect of Dorzolamide/Timolol Eye Drops, Solution b.i.d. was greater than that of monotherapy with either 2% dorzolamide t.i.d. or 0.5% timolol b.i.d. The IOP-lowering effect of Dorzolamide/Timolol Eye Drops, Solution b.i.d. was equivalent to that of concomitant therapy with dorzolamide b.i.d. and timolol b.i.d. The IOP-lowering effect of Dorzolamide/Timolol Eye Drops, Solution b.i.d. was demonstrated when measured at various time points throughout the day and this effect was maintained during long-term administration.
Paediatric population
A 3 month controlled study, with the primary objective of documenting the safety of 2% dorzolamide hydrochloride ophthalmic solution in children under the age of 6 years has been conducted. In this study, 30 patients under 6 and greater than or equal to 2 years of age whose IOP was not adequately controlled with monotherapy by dorzolamide or timolol received Dorzolamide/Timolol Eye Drops, Solution in an open label phase. Efficacy in those patients has not been established. In this small group of patients, twice daily administration of Dorzolamide/Timolol Eye Drops, Solution was generally well tolerated with 19 patients completing the treatment period and 11 patients discontinuing for surgery, a change in medication, or other reasons.
5.2 Pharmacokinetic properties
Dorzolamide hydrochloride
Unlike orally administered carbonic anhydrase inhibitors, topical administration of dorzolamide hydrochloride allows the drug to exert its effects directly in the eye at a significantly lower dose and hence with less systemic exposure. In clinical studies, this resulted in a reduction in intraocular pressure without acid-base imbalances or the electrolyte shifts characteristic of orally administered carbonic anhydrase inhibitors.
Following topical administration, dorzolamide reaches the systemic circulation. To assess possible systemic carbonic anhydrase inhibition after topical administration, drug and metabolite concentrations in red blood cells and plasma were measured, as well as carbonic anhydrase inhibition in red blood cells. During maintenance therapy, dorzolamide accumulates in red blood cells as a result of selective binding to carboanhydrase II (CA-II), whilst extremely low concentrations of the free drug remain in plasma.
Although the parent drug forms a single N-desethyl metabolite that inhibits carboanhydrase II (CA-II) less potently than the parent drug, it also inhibits another less active isoenzyme (CA-I). The metabolite also accumulates in red blood cells, where it binds mainly to CA-I. Dorzolamide displays moderate plasma protein binding (approximately 33%) and is mainly eliminated unchanged in the urine; the metabolite is also excreted in the urine. Once administration has ended, dorzolamide is washed out of red blood cells in a non-linear manner, initially resulting in a rapid decline in concentration, followed by a slower elimination phase with a half-life of approximately four months.
Following oral administration of dorzolamide to simulate maximum systemic exposure after long-term use of the topical ophthalmic form, steady state was reached within 13 weeks. At steady state, neither the free drug nor metabolites were detectable in plasma; carboanhydrase inhibition in red blood cells was less than that deemed necessary for a pharmacological effect on renal function or respiration. Similar pharmacokinetic results were observed following topical maintenance therapy with dorzolamide hydrochloride. However, whilst some elderly patients with renal dysfunction (estimated creatinine clearance 30-60 ml/minute) showed higher metabolite concentrations in red blood cells, the findings revealed no significant differences with regard to carbonic anhydrase inhibition and no clinically significant systemic adverse effects.
Timolol maleate
Aqueous humor level: In rabbits maximum aqueous humor levels of 461 ng/100 mg were measured 60 minutes after administering 1 drop of timolol 1.0%. In humans the aqueous humor levels of timolol during the 1st and 2nd hours after administering 2 drops of timolol 0.5 % were 150 ng/100 mg. After 7 hours had elapsed, the level fell to 10 ng/100 mg.
Ocular tissue level:
After applying one drop of a 0.25 % solution of 14C-marked timolol, the different ocular tissues of the rabbit eye reached maximum radioactivities after 15 to 60 minutes. The cornea, nictitating membrane and iris/ciliary body yielded radioactivities corresponding to 1 to 10 ng timolol/100 mg tissue.
Systemic resorption:
Studies have shown that after local application to the eye timolol is systemically resorbed. In one study timolol was detected in the urine of all examined healthy subjects and patients. (Timolol hydrogen maleate and its metabolites are primarily excreted by the kidneys.)
Blood level:
Blood levels of timolol in humans after local application to the eye at the recommended clinical dose frequently cannot be detected (smaller than 2 ng/ml), neither after a single dose nor after a treatment period of two weeks. The maximum measured plasma levels were 9.6 ng/ml at a dose of 2 x 2 drops/day. The maximum plasma levels were achieved after 30 -90 minutes.
It was shown in several cases that the application of timolol-containing eye drops in neonates and toddlers at the recommended dose led to significantly higher plasma concentrations of timolol than in adults. The plasma level in a three-week old neonate after administering 2 x daily 1 drop 0.25% timolol-containing eye drops was 34 ng/ml.
5.3 Preclinical safety data
The ocular and systemic safety profile of the individual active substances is sufficiently well-known.
Effects in non-clinical studies were observed only at exposures considered sufficiently in excess of the maximum human exposure indicating little relevance to clinical use.
Dorzolamide
With use in rabbits of maternotoxic doses associated with metabolic acidosis, vertebral body malformations were observed.
Timolol
Preclinical effects were observed after exposure to timolol, if the administered amount was sufficiently above the maximum therapeutic dose for humans. The relevance for humans is considered to be minimal.
Animal studies have not shown a teratogenic effect.
Furthermore, no adverse ocular effects were seen in animals treated topically with Dorzolamide hydrochloride and Timolol maleate ophthalmic solution or with concomitantly-administered Dorzolamide hydrochloride and Timolol maleate. In vitro and in vivo studies with each of the components did not reveal a mutagenic potential. Therefore, no significant risk for human safety is expected with therapeutic doses of Dorzolamide/Timolol Eye Drops, Solution.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Benzalkonium chloride
Hydroxyethylcellulose
Mannitol
Sodium citrate
Sodium hydroxide (for pH adjustment)
Water for injections
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
Shelf life: 24 months
Shelf life after first opening: 28 days
6.4 Special precautions for storage
Do not store above 25°C.
Keep the bottle in the outer carton in order to protect from light.
6.5 Nature and contents of container
Low density polyethylene (LDPE) bottle with LDPE dropper tip and polypropylene (PP) cap containing 5 ml solution.
Dorzolamide/Timolol Eye Drops, Solution is available in the following pack sizes:
Pack of 1 bottle of 5 ml Pack of 3 bottles of 5 ml each Pack of 6 bottles of 5 ml each
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
No special requirements.
7 MARKETING AUTHORISATION HOLDER
Bausch & Lomb UK Ltd 106 London Road Kingston-Upon-Thames Surrey KT2 6TN UK
8 MARKETING AUTHORISATION NUMBER(S)
PL 03468/0040
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
09/05/2011
10 DATE OF REVISION OF THE TEXT
29/03/2012