Asmavent Cfc-Free Inhaler 100 Micrograms
I
PATIENT INFORMATION LEAFLET
Asmavent ® CFC-free Inhaler 100 micrograms
Salbutamol (as sulphate)
Read all of this leaflet carefully before you start using this medicine.
- The full name of this product is Asmavent® CFC-free Inhaler 100 micrograms. However this name will be shortened within the text of this leaflet to Asmavent® CFC-free Inhaler.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, asthma nurse or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist. See Section 4
In this leaflet:
1. What Asmavent® CFC-free Inhaler is and what it is used for
2. Before you use Asmavent® CFC-free Inhaler
3. How to use Asmavent® CFC-free Inhaler
4. Possible side effects
5. How to store Asmavent® CFC-free Inhaler
6. Further information
1. WHAT ASMAVENT® CFC-FREE INHALER IS AND WHAT IT IS USED
FOR
Asmavent® CFC-free Inhaler is a pressurised inhalation suspension (inhaler) which contains the active ingredient salbutamol (as salbutamol sulphate). Salbutamol belongs to a group of medicines called short-acting l^agonists, bronchodilators or “relievers”. Salbutamol is used by patients with asthma as it helps to relieve the symptoms of asthma such as wheezing, shortness of breath, tightness in the chest and cough. Salbutamol is also used to prevent symptoms of asthma which are brought on by exercise or allergens such as house dust mite, pollen, cigarette smoke, cat and dog fur, etc.
Salbutamol can also be used to relieve symptoms such as chest tightness, wheezing, shortness of breath and coughing in some other chest diseases.
Salbutamol acts directly on the muscles in the walls of the airways in the lung causing the muscles to relax. This widens or opens up your airways making it easier to breathe. Asmavent® CFC-free Inhaler cannot be used with any spacing device at this time. If you need a spacing device, your doctor will need to prescribe another product, which can be used with a spacing device, instead of Asmavent ® CFC-free Inhaler.
2. BEFORE YOU USE ASMAVENT® CFC-FREE INHALER
Do not use Asmavent® CFC-free Inhaler if you are:
• allergic to salbutamol sulphate or any of the other ingredients in the inhaler (see Section 6).
• in premature labour or have a threatened miscarriage.
Tell your doctor or pharmacist before starting to take this medicine if you:
• are pregnant, think you may be pregnant, or trying to become pregnant. Tell your doctor or pharmacist before using Asmavent® CFC-free Inhaler.
• are breast-feeding; salbutamol may pass into your breast milk.
• are being treated for an overactive thyroid (thyrotoxicosis).
• are being treated for high blood pressure.
• have an irregular or very fast heartbeat / rhythm.
• are being treated for an irregular or very fast heartbeat / rhythm.
Taking other medicines
Please tell your doctor, pharmacist or asthma nurse if you are taking, or have recently taken, any other medicines, including other inhalers, or any medicines and tablets obtained without a prescription.
Tell your doctor, pharmacist or asthma nurse if you are taking any of the following medicines before using Asmavent® CFC-free Inhaler:
• beta-blockers (used to treat high blood pressure)
• disulfiram (used to treat alcoholism)
• metronidazole (used to treat some bacterial infections).
• xanthine derivatives for example aminophylline, theophylline tablets and inhaled steroids or steroid tablets (also used in the treatment of asthma)
• diuretics (water tablets)
• long-term laxatives.
Xanthine derivatives, steroids, diuretics and laxatives can all cause the level of potassium in your blood to fall. Your doctor will wish to monitor this and therefore from time to time may need to carry out a blood test to check your potassium levels.
Pregnancy, breast-feeding and fertility
The safe use of salbutamol during pregnancy has not been established. Tell your doctor if you are pregnant, if you think that you may be pregnant or if you are trying to become pregnant. Your doctor will advise you as to whether you should use Asmavent® CFC-free Inhaler or not.
Do not breast-feed unless your doctor advises you to.
Do not use Asmavent® CFC-free Inhaler if you are in premature labour or have a threatened miscarriage. Unlike salbutamol injection (and occasionally salbutamol tablets) inhaled salbutamol cannot be used to treat premature labour or threatened miscarriage. There is no information on the effects of salbutamol on human fertility.
There were no adverse effects on fertility in animals
Ask your doctor, asthma nurse or pharmacist for advice before taking any medicines, tablets or inhalers if you are pregnant of breast-feeding.
If you need to see another doctor or need to go i nto hospital, you should take all your medicines (including all your inhalers and any medicines or tablets bought without a prescription) with you, in the original packaging if possible.
Driving and using machines
It is unlikely that taking Asmavent® CFC-free Inhaler will have an effect on your ability to drive or operate machinery.
3. HOW TO USE ASMAVENT® CFC-FREE INHALER
Always use the inhaler exactly as your doctor or asthma nurse has told you to and take the recommended dose.
Make sure you know HOW, WHEN and HOW MANY puffs you need to take. This information should be on the pharmacist's label on the carton in which you received your_
inhaler. If it is not, or you are not sure, ask your doctor, pharmacist or asthma nurse. Asmavent® CFC-free Inhaler produces a fine mist, which must be inhaled through your mouth into your lungs. Make sure that you know how to use this inhaler properly, by reading the section “How to use your inhaler” later in this leaflet. If you have any problems ask your doctor, pharmacist or asthma nurse.
If you have changed to this inhaler, you may find that it tastes different from your previous inhaler.
Children
Asmavent® CFC-free Inhaler is not recommended for use in children 12 years of age and under.
Adults (including the Elderly)
For the relief of acute asthma symptoms (such as wheezing, shortness of breath or tightness in your chest):
Take one puff, as described in “How to use your inhaler”. This may be increased to 2 puffs if necessary.
For the prevention of symptoms due to exercise and due to allergens (e.g. house dust mite, pollen, cigarette smoke, animal fur etc.):
Take two puffs, as described in “How to use your inhaler”, 10 - 15 minutes before exercise or allergen exposure.
These are the usual doses. Your doctor may have told you to take a different dose, because all patients are different. It is very important that you follow your doctor's instructions carefully.
Asmavent® CFC-free Inhaler should be used as required.
However, do not take more than 8 puffs in 24 hours and do not take more than 2 puffs in 4 hours. If you find that you need to use your Asmavent® CFC-free Inhaler regularly every day or if you need to take more puffs than usual or you find that the dose is becoming less effective than usual, it may mean that your asthma is not very well controlled or is getting worse. You should contact your doctor or your asthma nurse straightaway.
If you find that your Asmavent® CFC-free Inhaler does not provide you with relief from your symptoms for at least 3 hours you should tell your doctor or your asthma nurse as soon as possible.
If your symptoms are getting worse, your doctor may tell you to take more puffs than usual as an emergency treatment. IT IS VERY IMPORTANT that you follow your doctor's instructions on how, when and how many puffs to take. You should contact your doctor or asthma nurse immediately if your symptoms are getting worse.
If you need to go into hospital, remember to take your inhaler with you.
How to use your inhaler:
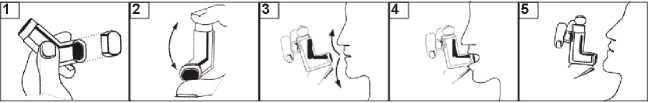
1.If your inhaler is new, or if you have not used your inhaler for a week or more, shake well, remove the mouthpiece cover and release two sprays into the air before using.
2. Remove the mouthpiece cover and check that the inside and outside of the mouthpiece is clear of dust, dirt or foreign objects (figure 1).
3. If the inhaler is very cold, the canister should be taken out of the plastic actuator and warmed in your hands for a few minutes before you use it. Do not use anything else to warm the canister. Shake the inhaler before each use (figure 2).
4. Hold the inhaler upright with a thumb on the base. Breathe out as far as is comfortable (it is important that you practice this before using the inhaler - see “Breathing technique” (figure 3).
5. and then immediately place the mouthpiece in your mouth and close your lips around it (figure 4). Be careful not to bite the mouthpiece.
6. Breathe i n slowly through your mouth. J ust after starting to breathe in through your mouth, press firmly down on the top of the inhaler to release an actuation (puff). Carry on breathing in deeply and steadily (figure 4).
7. Hold your breath, take the inhaler from your mouth and take your finger away from the top of the inhaler. Continue holding your breath for about 10 seconds, or for as long as is comfortable (figure 5). Then breathe out slowly.
8. If you are taking another puff, keep the inhaler upright and wait for at least 30 seconds before repeating steps 3-7.
9. After use, replace the mouthpiece cover firmly, making sure it snaps into position. Breathing Technique
You must breathe in as slowly as possible just before using the inhaler. Do not rush steps 5 to 7. You should practice a few times in front of a mirror. If you see “mist” coming from the inhaler or the sides of your mouth, then you need to start again from step 3.
People with weak hands may find it easier to hold the inhaler with two hands, with the two forefingers on the top of the inhaler and both thumbs on the bottom under the mouthpiece.
Cleaning vour inhaler
You should follow the cleaning instructions described below very carefully in order to ensure that your inhaler continues to work properly.
Clean your inhaler once a week, or if blocked.
1. First remove the metal can from the plastic actuator and take off the mouthpiece cover.
2. Rinse the plastic actuator, mouthpiece and mouthpiece cover in tap water. DO NOT place the metal can into water or clean the can using water. Make sure the water runs through the actuator from both ends to ensure that the actuator orifice (the small hole that can be seen through the mouthpiece) is clear and not blocked.
3. The plastic components (actuator and mouthpiece cover) should be placed in a warm place to dry thoroughly before putting the inhaler back together. Avoid drying near direct or excessive heat.
If you use more of your Asmavent® CFC-free Inhaler than you should
If you accidentally use too many puffs, you may feel shaky, have a fast heartbeat, headache, feel tense or experience flushing. These effects usually wear off within a few hours but if they do not wear off or are troublesome you must tell your doctor as soon as possible or go to your nearest hospital casualty department.
Low levels of potassium in the blood may also occur if you take too much salbutamol and therefore your doctor may want to do some blood tests to monitor your potassium levels. Do not use more than 2 puffs in 4 hours and never use more than 8 puffs in 24 hours. If you have any further questions on the use of this product, ask your doctor, asthma nurse or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Asmavent® CFC-free I nhaler can cause side effects, although not everybody gets them.
Side effects may include:
Common (seen in less than 1 in 10 people and more than 1 in 100 people):
• tremor (shakes - especially noticeable in your hands)
• headache
• rapid heartbeat (with or without flushing)
Uncommon (seen in less than 1 in 100 people and more than 1 in 1000 people):
• palpitations (faster or stronger heartbeats) or changes in your heartbeat (uneven or extra beats)
• changes in the rate or rhythm of the heartbeat
• mouth and throat irritation
• muscle cramps
Rare (seen in less than 1 in 1000 people and more than 1 in 10,000 people):
• low potassium levels in blood. Since salbutamol can change the salt balance in your body, your doctor may occasionally need to take a blood sample to check the potassium levels.
• flushing
Very rare (occurring in less than 1 in 10,000 people):
• Allergic reactions, which might include any of the following:
rapid swelling of the mouth, lips, tongue, face, ears, neck or throat (angioedema)
itching, raised bumps and/or redness on the skin (urticaria)
constriction of the small air passages in the lungs (bronchospasm)
low blood pressure (hypotension)
collapse
• Cardiac Arrythmias
• Restlessness or excitability (hyperactivity - particularly in children)
Unknown: Myocardial Ischaemia
There is some evidence from post-marketing data and published literature of rare occurrences of myocardial ischaemia associated with salbutamol.
If your breathing or wheezing gets worse immediately after using your inhaler, then stop using it immediately and contact your doctor straightaway. You may need to use a different “reliever” inhaler to treat your symptoms.
Patients with underlying severe heart disease (e.g. ischaemic heart disease, arrhythmia or s evere h eart f ailure) who ar e r eceiving s albutamol contact y our doctor if t hey experience chest pain or other symptoms of worsening heart disease.
If the relief of your symptoms is not as good as usual, or it does not last as long as usual, tell your doctor as soon as possible. This might mean that your asthma is getting worse and your treatment needs to be changed.
If any of the side effects get worse or become severe, or if you have any side effects not listed in this Leaflet, or if you are concerned in any way, please tell your doctor, asthma nurse or pharmacist.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme, website www.mhra.qov.uk/vellowcard
5. HOW TO STORE ASMAVENT® CFC-FREE INHALER
Keep out of the reach and sight of children.
Do not use Asmavent® CFC-free Inhaler after the “EXP” date which is stated on the canister and carton. The “EXP” date refers to the last day of that month.
Do not store above 30° C.
The canister contains a pressurised liquid. Do not expose the canister to temperatures higher than 50°C. Do not pierce the canister.
Do not dispose of the canister by burning. All unused or partially used and any unwanted inhalers should be r eturned to your pharmacist for disposal. Medicines should not be disposed of via wastewater or household waste. These measures will help to protect the environment.
6. FURTHER INFORMATION
What Asmavent® CFC-free Inhaler looks like and contents of the pack
The salbutamol inhaler consists of an aluminium canister sealed with a metering valve. The canister is held in a blue plastic actuator fitted with a removable blue plastic mouthpiece cover.
Each canister contains 200 actuations (puffs). Each actuation (puff) contains 100 micrograms of salbutamol (as sulphate).
The other ingredients are oleic acid, ethanol and norflurane (HFA 134a; a CFC-free propellant).
Marketing Authorisation Holder:
Fannin (UK) Limited, 42-46 Booth Drive, Park Farm South, Wellingboroughn,eoiab Northamptonshire, NN8 6GT, UK.
Manufacturer responsible for batch release: Fannin (UK) Limited,
42-46 Booth Drive, Park Farm South, Wellingborough, Northamptonshire,
NN8 6GT, UK.
This leaflet was last approved in Jan 14