Astepro 0.15% Nasal Spray
Out of date information, search anotherPackage leaflet: Information for the user
Astepro 0.15% Nasal Spray
Azelastine hydrochloride
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
- In this leaflet Astepro 0.15% Nasal Spray is called Astepro.
What is in this leaflet:
1. What Astepro is and what it is used for
2. What you need to know before you use Astepro
3. How to use Astepro
4. Possible side effects
5. How to store Astepro
6. Contents of the pack and other information
1. What Astepro is and what it is used for
Astepro contains azelastine hydrochloride which belongs to a group of medicines called antihistamines. Antihistamines work by preventing the effects of histamine that the body produces as part of an allergic reaction.
Astepro is used to treat allergic rhinitis in adults, adolescents and children 6 years and older. This is an allergic reaction to substances such as, for example, pollen, house dust mites or animal hair.
Usually it affects you by causing a runny nose, sneezing, itching or blocked nose. Astepro should help control these symptoms.
2. What you need to know before you use Astepro
Do not use Astepro
- if you are allergic (hypersensitive) to azelastine hydrochloride or any of the other ingredients of
this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before using Astepro.
Children
Astepro Nasal Spray is not recommended for use in children below 6 years of age.
Other medicines and Astepro
Even if Astepro is not known to be affected by other medicines, tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Pregnancy and breast-feeding
There are only limited information on the effects of Astepro on the unborn or breast fed child.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
Astepro Nasal Spray has minor influence on the ability to drive and use machines.
Rarely, you may experience fatigue or dizziness due to the disease itself or when using Astepro. In these cases, do not drive or operate machinery. Please be aware that drinking alcohol may enhance these effects.
Astepro contains benzalkonium chloride
Astepro contains the preservative benzalkonium chloride which is an irritant and may cause skin reactions.
3. How to use Astepro
Always use this medicine exactly as your doctor or pharmacist has told you. Astepro is for nasal use. Important:
If you are not sure of the correct dose or how to use this medicine, ask your doctor or pharmacist.
Adults and adolescents 12 years and older:
• The recommended dose is two sprays in each nostril once daily. In some cases two sprays in each nostril twice daily may be required.
• The maximum daily dose is two sprays in each nostril twice daily.
Children 6 to 11 years:
• The recommended dose is one spray in each nostril twice daily.
Clinical experience of up to 4 weeks duration showed good efficacy and safety in children. Longer experiences in children have not been available; however, clinical trials of up to one year duration using a double higher daily dose showed good safety in adults and adolescents.
Astepro is not recommended for the use in children under 6 years of age due to a lack of data on safety and/or efficacy.
If possible, you should use Astepro regularly until your symptoms have disappeared. If you interrupt the use of Astepro your symptoms are likely to return.
Astepro is suitable for long-term use.
Use longer than 4 weeks is not recommended in children 6-11 years due to lack of clinical data.
How to use the spray
1. Blow your nose first.
2. Remove the protective cap (Diagram 1).
3. Before the first use, press the pump six times until an even spray emerges (Diagram 2). When Astepro Nasal Spray has not been used for three or more days, the pump must be pressed a sufficient number of times until a fine mist emerges.
4. Spray once into each nostril keeping head upright. Do not tilt head backwards (Diagram 3).
5. Wipe and replace the protective cap.
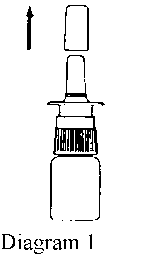
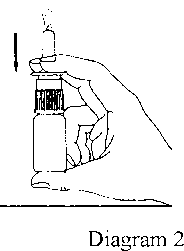
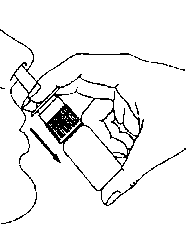
Diagram 3
If you use more Astepro than you should
If you spray too much Astepro into your nose you are unlikely to have any problems. If you are worried, contact your doctor.
If anyone, especially a child, accidentally drinks Astepro, contact your doctor or nearest hospital casualty department immediately.
If you forget to use Astepro
Use the spray as soon as you remember, then take the next dose at the usual time. Do not take a double dose to make up for a missed dose.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Like all medicines, this medicine can cause side effects, although not everybody gets them.
These effects include:
Common (may affect up to 1 in 10 people):
- An unpleasant taste in your mouth (especially if you tilt your head backwards when you are using the nasal spray).
Uncommon (may affect up to 1 in 100 people):
- Slight irritation of the inside of the nose (stinging, itching), sneezing and nose bleed.
Rare (may affect up to 1 in 1,000 people):
- The unpleasant taste might cause you to feel sick. Fatigue (weariness, exhaustion), dizziness, weakness or a feeling of drowsiness can occur that may also be caused by the disease itself.
Very rare (may affect up to 1 in 10,000 people):
- An allergic reaction, severe allergic reaction, rash, itching or nettle rash.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme (www.mhra.gov.uk/yellowcard). By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Astepro
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date given on the bottle label and the outer carton. The expiry date refers to the last day of the month.
Dispose of any unused medicine 6 months after you first open the nasal spray.
Do not store the spray in the fridge or freezer.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Astepro contains
The active substance is azelastine hydrochloride.
1 ml of nasal spray contains 1.5 mg azelastine hydrochloride. Each squirt (0.14 ml) contains 0.21 mg azelastine hydrochloride equivalent to 0.19 mg azelastine.
The other ingredients are hypromellose, sucralose, sorbitol, liquid (crystallising), disodium edetate, sodium citrate, benzalkonium chloride and purified water.
What Astepro looks like and contents of the pack
Astepro Nasal Spray is a clear, colourless solution.
It comes in a white HDPE plastic bottle fitted with a spray pump. The 15 ml bottle contains 4 ml of the nasal spray. The 34.5 ml bottle contains 30 ml of the nasal spray. Not all pack sizes may be marketed.
Marketing Authorisation Holder
Meda Pharmaceuticals Limited
Skyway House
Parsonage Road
Takeley, Bishop’s Stortford
UK
Manufacturer
MEDA Pharma GmbH & Co. KG Benzstrasse 1 61352 Bad Homburg Germany
This medicinal product is authorised in the Member States of the EEA under the following names:
Austria
Denmark
Finland
Germany
Ireland
Italy
Spain
The Netherlands
Portugal
Sweden
United Kingdom
Astepro1.5 mg/ml Nasenspray Astepro
Astepro 0.19 mg/annos nenasumute, liuos Astepro 1.5 mg/ml Nasenspray Astepro 1.5 mg/ml Nasal Spray, Solution Astepro 0.15 %
Astepro 1,5 mg/ml solution para pulverization nasal
Asteopro 0.15 %
Allergodil BID 0,15%
Astepro 0.19 mg/dos nasspray, losning Astepro 0.15 % nasal spray
This leaflet was last revised in August 2013