Azelastine Hydrochloride 0.5Mg/Ml Eye Drops Solution
Out of date information, search another|
MICRO Ban |
LABS L galore, IN |
IMITED DIA |
Azelastine hydrochloride 0.5mg/ml Eye drops, ) p ' solution Leaflet (UK) 2) No. of Colours : 1 3) Dimension : 120 x 420 mm 4) Pharma Code : NA 5) Font & Size : Arial Narrow, 9 pt |
Colours used Black Keyline - NOT TO PRINT | ||||
|
WOA Code No. : WA |
Artwork code : NA |
Revision : 00 Dated : 11-01-13 | ||||||
|
Client Approval |
Packaging Development |
Quality Assurance (QA) |
Quality Control (QC) |
Production | ||||
▲
PACKAGE LEAFLET: INFORMATION FOR THE USER
Azelastine hydrochloride 0.5mg/ml Eye drops, solution Azelastine hydrochloride
Read all of this leaflet carefully before you start taking this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Azelastine hydrochloride Eye drops is and what it is used for
2. Before you use Azelastine hydrochloride Eye drops
3. How to use Azelastine hydrochloride Eye drops
4. Possible side effects
5. How to store Azelastine hydrochloride Eye drops
6. Further information
BROWN & BURK
Azelastine hydrochloride Eye drops contains an antihistamine called azelastine hydrochloride. This compound prevents the effects of histamine (and other substances that the body produces), which are produced as part of an allergic reaction. Azelastine has been shown to reduce inflammation of the eye.
Azelastine hydrochloride Eye drops is used to treat and prevent both seasonal allergic conjunctivitis (hay fever, which is allergy to pollen) and perennial allergic conjunctivitis (which is allergy to other substances, such as house dust mites and animal dander, and which can occur at any time of the year). The symptoms of these conditions are red, itchy and/or watery eyes, sometimes together with sneezing or a runny, itchy or blocked nose. Azelastine hydrochloride Eye drops should help with the eye symptoms.
Azelastine hydrochloride Eye drops can be used to treat and prevent eye disorders which you get with hayfever (seasonal allergic conjunctivitis) in adults and children age 4 years and above.
Azelastine hydrochloride Eye drops can be used for eye disorders caused by an allergy to substances such as house dust mites or animal hair (perennial allergic conjunctivitis) in adults and children age 12 years and above.
Azelastine hydrochloride Eye drops is not suitable for treating eye infections.
Do not use Azelastine hydrochloride Eye drops:
• If you have ever had an allergic reaction to azelastine hydrochloride, benzalkonium chloride (a preservative) or any of the other ingredients of azelastine hydrochloride eye drops (see section 6).
Warnings and precautions
Talk to your doctor or pharmacist before using Azelastine hydrochloride Eye drops.
• if you are not sure whether your eye disorders are caused by an allergy, in particular if only one eye is affected; if your vision is impaired; or the eye hurts and you do not have any symptoms in your nose, you may have an infection rather than an allergy.
• if the complaints worsen or last longer than 48 hours without remarkable improvement despite the use of Azelastine hydrochloride Eye drops.
• If you see any other doctor or go to hospital for treatment, tell your doctor about all your medicines, including Azelastine hydrochloride Eye drops.
Azelastine hydrochloride Eye drops is not recommended for use whilst wearing contact lenses. Azelastine hydrochloride Eye drops contains the preservative benzalkonium chloride which is known to discolour soft contact lenses. Avoid contact with soft contact lenses. Remove contact lenses prior to application and wait at least 15 minutes before reinsertion.
Azelastine hydrochloride Eye drops is not intended for the treatment of eye infections.
Azelastine hydrochloride Eye drops contains a preservative called benzalkonium chloride to which you may be allergic.
Azelastine hydrochloride Eye drops should only be applied to the eyes, do not drink it.
Azelastine hydrochloride Eye drops should not be used for more than 6 weeks when used to treat perennial allergic conjunctivitis.
Other medicines and Azelastine hydrochloride Eye drops
Azelastine hydrochloride Eye drops is not known to be affected by other medicines.
Azelastine hydrochloride Eye drops with food and drink
Azelastine hydrochloride Eye drops is not known to react with any types of food and drink.
Pregnancy and breast-feeding
If you are pregnant, trying to become pregnant or breast-feeding ask your doctor or pharmacist for advice before using Azelastine hydrochloride Eye drops.
Driving and using machines
Like other eye drops, you may have a transient blurring of vision after instilling the eye drops. You should wait until this clears before driving or operating machinery.
Always use Azelastine hydrochloride Eye drops exactly as your doctor has told you.
Important:
• If you are not sure of the correct dose or how to use this medicine, ask your doctor or pharmacist.
Eye disorders caused by hayfever (seasonal allergic conjunctivitis)
• Use in adults and children aged 4 years and above
• The usual dose is one drop in each eye in the morning and evening.
If you anticipate contact with pollen, the usual dose of Azelastine hydrochloride Eye drops may be taken as a preventive measure before going outside.
Eye disorders caused by an allergy (non-seasonal (perennial) allergic conjunctivitis)
• Use in adults and children aged 12 years and above
• The usual dose is one drop in each eye in the morning and evening.
If your symptoms are severe, your doctor may increase your dose to one drop in each eye, up to four times a day.
▼
If possible, you should use Azelastine hydrochloride Eye drops regularly until your symptoms have disappeared.
If you interrupt the use of Azelastine hydrochloride Eye drops your symptoms are likely to return. Remember:
• Do not take Azelastine hydrochloride Eye drops for more than 6 weeks
• Azelastine hydrochloride Eye drops should only be applied to the eyes.
Opening your Azelastin hydrochloride Eye drops for the first time:
These eye drops come in a bottle with a screw cap with shrink wrap around the cap extending upto shoulder of the container. You must not use the bottle if the tamper-proof shrink wrap is broken before you first use it. The inside of the screw cap contains a spike. The screw cap is fitted to the bottle in such a way that there is no contact between the spike and the top of the bottle, In order to open the bottle it is necessary to remove the tamper-proof shrink wrap and tighten the screw cap further, so that the edge of the screw cap and the bottle edge are totally aligned. At this point the screw cap spike will pierce the top of the bottle which will open the bottle. Once the bottle has been opened in this way, unscrew the screw cap fully to remove it from the bottle and apply the eye drops.
Putting in your Azelastine hydrochloride Eye drops
To help you put in your eye drops correctly, you may find it useful to sit in front of a mirror so you can see what you are doing for the first few times.
1. Wash your hands.
2. Gently wipe around your eyes with a tissues to remove any moisture (Diagram 1).
3. Unscrew the top of the bottle and check that the dropper is clean.
4. Gently pull your lower eye lid down (Diagram 2).
5. Carefully place the drop inside the middle of your lower eyelid (Diagram 3). Take care not to let the dropper touch your eye.
6. Release your lower eye lid and gently press on the inner corner of your eye against the bridge of your nose (Diagram 4). Keeping your finger pressed against your nose, slowly blink your eyes a few times to spread the drop across the surface of your eye.
7. Blot away any excess medicine with a tissue.
8. Repeat this for your other eye.
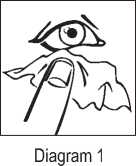
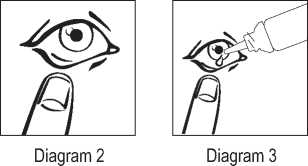

If you use more Azelastine hydrochloride Eye drops than you should
If you put too much Azelastine hydrochloride Eye drops into your eyes you are unlikely to have any problems. If you are worried, contact your doctor. If you accidentally swallow Azelastine hydrochloride Eye drops, contact your doctor or nearest hospital casualty department as soon as possible.
If you forget to use Azelastine hydrochloride Eye drops
Use your eye drops as soon as you remember, then take the next dose at the usual time. Do not take a double dose to make up for a missed dose. If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
Like all medicines Azelastine hydrochloride Eye drops can cause side effects. Not everybody gets
them and they are not usually serious.
These effects include:
• Common (1 to10 of 100 patients treated): Slight irritation (burning, itching, watering) in the eyes after putting in Azelastine hydrochloride Eye drops. This should not last long.
• Uncommon (1 to 10 of 1,000 patients treated): A bitter taste in your mouth. This should quickly disappear especially if you have a soft drink.
• Very rare (less than 1 of 10,000 patients treated): An allergic reaction (such as rash and itching).
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep Azelastine hydrochloride Eye drops in a safe place where children cannot see or reach it. Used wrongly, your eye drops could harm them.
This medicinal product does not require any special storage conditions.
Do not use Azelastine hydrochloride Eye drops after the expiry date given on the bottle label and the outer carton. The expiry date refers to the last day of that month.
Once you have opened your bottle of Azelastine hydrochloride Eye drops, do not use it for longer than 4 weeks, even if there is some medicine left in the bottle after this time. Take your bottle back to a pharmacist for disposal of the unused eye drops and ask your doctor for a new prescription.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
What Azelastine hydrochloride Eye drops contains:
The active substance is azelastine hydrochloride 0.05% (0.5 mg/ml). Each drop (30pl) contains 0.015 mg azelastine hydrochloride.
• The other ingredients are benzalkonium chloride, disodium edetate, hypromellose, liquid sorbitol crystallising, sodium hydroxide and water.
What Azelastine hydrochloride Eye drops looks like and contents of the pack
Azelastine hydrochloride Eye drops is a clear, colourless, sterilised, water based solution which comes in a polyethylene bottle capped with polypropylene spiked cap with shrink wrap around the cap extending upto shoulder of the container. The bottle contains 6 ml, 8 ml or 10 ml of eye drops. Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Brown & Burk UK Ltd 5, Marryat Close Hounslow West Middlesex TW4 5DQ UK
This medicinal product is authorised in the Member States of the EEA under the following names:
UK: Azelastine hydrochloride 0.5mg/ml Eye drops, solution DE: Azelastinhydrochlorid-Brown 0.5 mg / ml Augentropfen IE: Azelastine hydrochloride 0.5mg/ml Eye drops, solution PL : Azelastine- Brown