Bcg Vaccine Ssi Powder And Solvent For Suspension For Injection

AJVaccines
5, Artillerivej DK-2300 Copenhagen S Denmark
Package leaflet: Information for the user
BCG Vaccine SSI
Powder and solvent for suspension for injection.
Mycobacterium bovis BCG (Bacillus Calmette-Guerin), Danish strain 1331, live attenuated.
Read all of this leaflet carefully before you are vaccinated because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others.
- If you get any side effects talk to your doctor, pharmacist or nurse.
This includes any possible side effects not listed in this leaflet.
See section 4.
What is in this leaflet
1. What BCG Vaccine SSI is and what it is used for
2. What you need to know before you are vaccinated with BCG Vaccine SSI
3. How you are vaccinated with BCG Vaccine SSI
4. Possible side effects
5. How to store BCG Vaccine SSI
6. Contents of the pack and other information
1. What BCG Vaccine SSI is and what it is used for
BCG Vaccine SSI contains bacteria of the type Mycobacterium bovis BCG and is used for protection against tuberculosis (TB).
2. What you need to know before you are vaccinated with BCG Vaccine SSI
You should not be vaccinated with BCG Vaccine SSI
• If you have known allergies to any of the excipients in the vaccine (listed in section 6)
• If you are suffering from an acute severe febrile illness or generalised skin infection. In these cases vaccination should be postponed
• If you have a weakened resistance toward infections due to a disease in/of your immune system
• If you are receiving medical treatment that affects the immune response e.g. corticosteroids or radiotherapy
• If you have been exposed to immunosuppressive treatment in utero or via breast-feeding (e.g. treatment with TNF-a antagonists)
• If you are suffering from any malignant conditions (e.g. lymphoma, leukaemia or Hodgkin’s disease)
• If your immune status is in question
• If you are infected with HIV
• If you are receiving medical treatment against TB
The doctor or nurse will be extra cautious about vaccinating you with BCG Vaccine SSI
• If you have eczema. The vaccination can be given in an eczema-free area
• If you have been skin tested for TB infection and the test was found positive vaccination is not required. Vaccination may cause a severe local reaction in that case
Other medicines and BCG Vaccine SSI
• Tell your doctor or pharmacist if you are taking, have recently taken or might take any others medicines
• Other vaccines can be given at the same time as BCG Vaccine SSI at different injection sites
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before being vaccinated with BCG Vaccine SSI.
Vaccination is not recommended during pregnancy or breast-feeding, although no harmful effects to the unborn or breastfed child have been associated with BCG Vaccine SSI.
Driving and using machines
BCG Vaccine SSI has no influence on the ability to drive and use machines.
3. How you are vaccinated with BCG Vaccine SSI
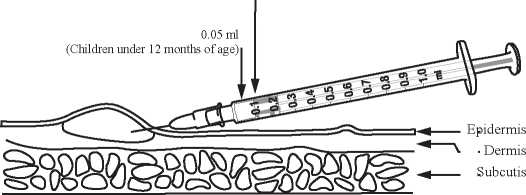
The doctor or nurse will give the vaccination by injection into the upper layer of the skin.
The dose is 0.05 ml for children under 12 months of age and 0.1 ml for adults and children aged 12 months or more.
The injection site is best left uncovered to facilitate healing.
The expected reactions to the vaccination include:
• a slight swelling, redness and tenderness at the injection site followed by a local lesion
• some weeks later this lesion evolves into a small ulcer
• after some months this ulcer will heal leaving a small, flat scar
• a slight swelling of the lymph nodes in the armpit may be experienced
These are common reactions to the vaccination.
4. Possible side effects
Like all medicines, BCG Vaccine SSI can cause side effects, although not everybody gets them.
Severe allergic reactions (such as redness of the face and neck, swelling of the face, throat or neck, skin rash, breathing difficulties and collapse) may occur in rare cases (less than 1 in 1,000).
If you observe any of the above reactions contact your doctor immediately.
The following information is intended for medical and healthcare professionals only
Special warnings and precautions for use
The vaccine should be administered strictly by the intradermal route.
The vaccine should preferably be administered by personnel trained in the intradermal vaccination technique.
Inadequate administered injections e.g. subcutaneously or intramuscularly increase the risk of lymphadenitis and abscess formation.
Tuberculin skin test positive persons should not be vaccinated as this may result in an aggravated loco-regional reaction.
Although anaphylactic reactions are rare, facilities for its management should always be available during vaccination. Whenever possible, persons should be kept under observation for 15-20 minutes after vaccination, in case an allergic reaction should occur.
BCG vaccination may be given concurrently with inactivated or live vaccines, including combined measles, mumps and rubella vaccines. If not given concurrently a period of not less than 4 weeks must pass before giving another live vaccine.
There must be an interval of at least 3 months before a vaccination in the same arm can take place.
Handling
The rubber stopper must not be wiped with any antiseptic or detergent. If alcohol is used to swab the rubber stopper, it must be allowed to evaporate before the stopper is penetrated with the syringe needle.
Using a syringe fitted with a long needle, transfer to the vial the volume of solvent stated on the label. Do not use other diluents, as these may damage the vaccine.
Carefully invert the vial a few times to the resuspend the lyophilised BCG completely.
Do not shake the vial. Gently swirl the vial with the reconstituted vaccine before drawing up each subsequent dose.
When drawn up into the syringe the reconstituted vaccine should appear homogeneous, slightly opaque and colourless. When reconstituted the vaccine should be used within 4 hours.
Method of administration
The vaccine should be administered by personnel trained in the intradermal technique.
The injection site should be clean and dry.
If antiseptics (such as alcohol) are applied to swab the skin, it must be allowed to evaporate before injection.
The vaccine must be given strictly intradermally, approximately one third down the upper arm corresponding to the area of the distal insertion of the deltoid muscle, as follows:
Other side effects include:
Uncommon side effects (may occur in less than 1 in 100 people)
• Fever
• Swelling of lymph nodes in the armpit larger than 1 cm across
• An oozing ulcer at the injection site
• Headache
Rare side effects (may occur in less than 1 in 1,000 people)
• Inflammation of lymph nodes, sometimes with oozing ulcers, possibly abscess
• Infection with the bacteria from the vaccine can occur. The infection can spread throughout the body, including the bones
Fainting, seizures and convulsions among patients receiving injections have been observed.
In babies born very prematurely (at or before 28 weeks of gestation) longer gaps than normal between breaths may occur for 2-3 days after vaccination.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store BCG Vaccine SSI
• Keep this medicine out of sight and reach of children
• Store in a refrigerator (2°C - 8°C)
• Store in the original package in order to protect from light
• Do not freeze
• Do not use the vaccine after the expiry date which is stated on the carton as “EXP”
• The expiry date refers to the last day of that month
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What BCG Vaccine SSI contains
The active substance is:
Freeze-dried powder containing live attenuated bacteria of the type Mycobacterium bovis BCG (Bacillus Calmette-Guerin), Danish strain 1331.
1 ml vaccine contains between 2-8 million bacteria.
The excipients are:
Sodium glutamate, magnesium sulphate heptahydrate, dipotassium phosphate, L-asparagine monohydrate, ferric ammonium citrate, glycerol 85%, citric acid, monohydrate and water for injections.
What BCG Vaccine SSI looks like and contents of the pack
BCG Vaccine SSI consists of a powder and solvent for suspension for injection (2-8 x 105 bacteria/0.1 ml dose or 1-4 x 105 bacteria/0.05 ml dose). Pack sizes of 1, 5, or 10 vials and pack size of 1 vial with one syringe and two injection needles (one long for adding solvent and one short for intradermal injection).
The powder in the amber vial is white and crystalline, the powder might be difficult to see due to the small amount of powder in the vial.
The solvent in the clear vial is a colourless solution without visible particles.
The mixed vaccine should appear as a homogenous, slightly opalescent, colourless suspension.
Pack sizes: 1, 5, 10-vial packages and 1 vial enclosed in a 1-dose injection kit.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
AJ Vaccines A/S 5, Artillerivej DK-2300 Copenhagen S Denmark
tel.: +45 7229 7000 fax: +45 7229 7999 e-mail: ajvaccines@ajvaccines.com
This medicinal product is authorised in the Member States of the EEA under the following names:
DK, EL, FI, SK, UK: BCG Vaccine SSI FR: VACCIN BCG SSI NO: BCG-vaksine SSI PL: BCG Szczepionka SSI SE: BCG-vaccin SSI
This leaflet was last revised in 11/2016
20-024-01
en
11-2016
• The skin is stretched between thumb and forefinger
• The needle should be almost parallel with the skin surface and slowly inserted (bevel upwards), approximately 2 mm into the superficial layers of the dermis. The needle should be visible through the epidermis during insertion
• The vaccine should be given slowly
• A raised, blanched papule at the needle point is a sign of correct injection
• The injection site is best left uncovered to facilitate healing
0.1 ml
(Adults and children aged 12 months or more)
Administering the vaccine too deep increases the risk of discharging ulcer, lymphadenitis and abscess formation.
Treatment of complications after vaccination with BCG Vaccine SSI
Expert advice should be sought regarding the appropriate treatment regimen for the management of systemic infections or persistent local infections following vaccination with BCG Vaccine SSI.
Antibiotic sensitivity of the BCG strain:
The table below indicates the minimum inhibitory concentrations (MIC) for selected anti-tuberculous drugs towards the BCG Danish strain 1331 [as determined by Bactec 460].
The MIC for isoniazid is 0.4 mg/l. There is no consensus as to whether Mycobacterium bovis BCG should be classified as susceptible, intermediately susceptible or resistant to isoniazid when the MIC is 0.4 mg/l. However, based on criteria set for Mycobacterium tuberculosis, the strain could be considered to be of intermediate susceptibility.
|
Drug |
Minimum Inhibitory Concentration (MIC) |
|
Isoniazid |
0.4 mg/l |
|
Streptomycin |
2.0 mg/l |
|
Rifampicin |
2.0 mg/l |
|
Ethambutol |
2.5 mg/l |
BCG Danish strain 1331 is resistant to pyrazinamide.
The mixed vaccine should be administered with a syringe of 1 ml graduated into hundredths of millilitre (1/100) fitted with a short bevel syringe needle (25G or 26G).
Jet injectors or multiple puncture devices should not be used to administer the vaccine.
Over dosage or incorrect administration
Overdose increases the risk of suppurative lymphadenitis and may lead to excessive scar formation.
Gross over dosage increases the risk of undesirable BCG complications.
en