Beclometasone Dipropionate Aqueous Nasal Spray
TEVA UK Ref: 231-30-83748-E LEA BECLOM (NASOBEC) 50MCG SPR TUK <OPA (P)
Version: 1 13 March 2015
PACKAGE LEAFLET: INFORMATION FORTHE USER
Read all of this leaflet carefully because it contains mportant information for you.
This medicine is available without prescription. However, you still need to use Nasobec Hayfever carefully to get the best results from it.
Keep this leaflet. You may need to read it again. Askyour pharmacist ifyou need more information or advice.
You should not use Nasobec Hayfeverfor more than 3 months continuously without consulting a doctor.
Ifyou get any side effects, talkto your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
INTHIS LEAFLET:
Driving and using machines
♦ Nasobec Hayfever is not expected to affect your ability to drive or operate machinery.
. What Nasobec Hayfever is and what it is used for
2. Before you use Nasobec Hayfever
3. How to use Nasobec Hayfever
4. Possible side effects
5. How to store Nasobec Hayfever
6. Further information
WHAT NASOBEC HAYFEVER IS AND WHAT IT IS USED FOR
Nasobec Hayfever contains beclometasone dipropionate, which is one of a group of medicines called corticosteroids. Your medicine has anti-inflammatory properties.
Nasobec Hayfever is used to treat and prevent the symptoms of allergic reactions to pollens (hayfever), such as itchy or runny nose and sneezing.
^ BEFORE YOU USE NASOBEC HAYFEVER
DO NOT use Nasobec Hayfever if you:
are allergic(hypersensitive)to beclometasone dipropionate or any of the other ingredients of this medicine ifyou are under 18 years.
Take special care with Nasobec Hayfever
Talkto your doctor of pharmacist before using Nasobec Hayfever if you have an infection or ulcers in your nose, or you have recently had surgery or an injury to your nose If your symptoms do not improve within 14 days, talkto your doctoror pharmacist Do not use for more than 3 months without consulting your doctor.
Taking other medicines
Please tell your doctoror pharmacist ifyou are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Important information about some of the ingredients of Nasobec Hayfever
* Nasobec Hayfever also includes the ingredient benzalkonium chloride, which is an irritant and may cause skin reactions.
Pregnancy and breast-feeding
Ifyou are pregnant, planning to become pregnant or are breast-feeding, ask yourdoctor or pharmacist for advice before using this medicine.
If your doctor has prescribed this medicine, always use Nasobec Hayfever exactly as your doctor has told you. Otherwise, follow the instructions below. You should checkwith yourdoctor or pharmacist if you are not sure.
Tell your doctor or pharmacist if you have watery or itchy eyes. He or she may give you another medicine to treat your eyes.
Always shake well before use. Only use in the nose. Do not swallow.
Adults (including the elderly) aged 18 and over
The usual dose is either:
♦ 2 sprays into each nostril twice a day, or if you
prefer
♦ 1 spray into each nostril 3 or 4 times a day.
Do not use more than eight sprays (400 micrograms) in 24 hours.
You will only start to feel better after you have been using your medicine for a few days.
Once your symptoms are under control you should change your dosage to 1 spray into each nostril twice a day.
It is very important to reduce your dose as soon as your symptoms are under control.
If your symptoms then get worse, you should increase your dosage backto the starting dose.
Do not use Nasobec Hayfever for more than 3 months continuously without consulting a doctor. Do not use more than the recommended dose.
If you use high doses of Nasobec Hayfever There are times when your doctor may need to adjust the dose of the steroid you are given or the way in which you take it.These times are:
♦ times of extreme stress
♦ during admission to hospital aftera serious accident, injury or illness
♦ before oraftera surgical operation.
If you think that any ofthese apply to you whilst using Nasobec Hayfever, speakto your doctor or pharmacist.
Children less than 18 years old
♦ Nasobec Hayfever is not recommended for use in children less than 18 years old.
Before you use your nasal spray
1. If you are using your nasal spray forthe first time,
remove the clip (diagram A).
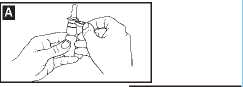
2. Shake the bottle gently and then remove the dust cap.
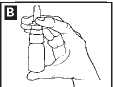
3. Hold the bottle upright with your thumb under the bottle and your index finger and middle finger on either side of the
nozzle (diagram B). .
4. To make sure your pump is working, test spray it into the air before you use it for the first time, and wheneveryou have not used itfora weekor
more. Hold the bottle away from you and press down until you can see a fine mist.
Using your nasal spray
5. Gently blow your nose.
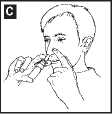
6. Close one nostril by pressing your finger against it and place the nozzle of the spray in your other nostril. Tilt your head slightly so that the bottle is kept upright (diagram C).
7. Breathe in through your open nostril and, at the same time, press the nozzle once with your fingers to release the spray.
8. Breathe out through your mouth.
9. If you need to use a second spray, repeat steps 7 and 8.
10. Repeat steps 6, 7, 8 and 9, with your other nostril. After using your nasal spray
Wipe the nozzle with a clean tissue and put the dust cap backon.
Cleaning your nasal spray
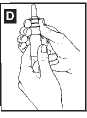
You should clean the pump at least once a week to stop it from getting blocked up. Gently press
upwards on the white collarto pull the nozzle off (diagram D)
Wash the nozzle and dust cap in warm water. Put them in a warm place to dry, but do not use too much heat. Put the nozzle and dust cap backon.
If you use more Nasobec Hayfever than you should It is important that you take your dose as stated on the carton and in the "How to use" section of this leaflet or as advised by your pharmacist.You should not exceed the recommended dose; using more or less may make your symptoms worse.
If you use more than 8 sprays in 24 hours, tell your doctor or pharmacist immediately.
If you forget to use Nasobec Hayfever If you forget to use your medicine at the right time, use it as soon as you remember, and then carry on as before. Never take two doses togetherto make up for a forgotten dose.
If you stop using Nasobec Hayfever Your symptoms may only start to improve after you have been using your medicine for a few days, therefore it is important that you use your medicine regularly for full benefit. If your symptoms do not improve after 7 days, consult your doctor before you stop using this medicine.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
^ POSSIBLE SIDE EFFECTS
Like all medicines, Nasobec Hayfever can cause side effects, although not everybody gets them.
Stop using the spray and tell your doctor or pharmacist immediately or go to the casualty department at your nearest hospital if the following happens: an allergic reaction (swelling ofthe lips, face or neck leading to severe difficulty in breathing; shortness of breath or wheezing, skin rash or hives).
This is a very serious but rare side effect. You may need urgent medical attention or hospitalisation.
Immediately after you use your spray
you may sneeze a little, but this soon stops
♦ very occasionally you may find you get an unpleasant taste or smell.
Tell your doctor as soon as possible if you notice any of the following side effects:
Common (affects less than 1 in 10 people)
♦ a dry or painful nose or throat
♦ bad nose bleeds
♦ mild allergic reactions including skin rashes or redness, itching or weals like nettle rash or hives.
Very rare (affects less than 1 in 10,000 people)
♦ high pressure in your eye that may cause pain or blurred vision
♦ clouding ofthe lens (cataract)
♦ damage to your nose
♦ the nose septum (the dividing partition in the nose) may become perforated.
The following side effects may occur:
♦ blood stained crusts in the nose.
It is important to follow the dose instructions shown in Section 3. Using Nasobec Hayfever at higher doses or for longer than 3 months at a time can cause a number of changes in your body, such as catching infections more easily.
Reporting of side effects
If you get any side effects, talkto your doctor,
pharmacist or nurse. This includes any possible side
effects not listed in this leaflet. You can also report
side effects directly via the Yellow Card Scheme at:
By reporting side effects you can help provide more
information on the safety of this medicine.
^ HOWTO STORE NASOBEC HAYFEVER
Keep out of the reach and sight of children.
Keep your medicine below 30°C in the original carton away from light. Do not put your medicine in the fridge. Do not use Nasobec Hayfever after it has been open for3 months orafter the expiry date that is stated on the outer packaging, whichever is sooner.The expiry date refers to the last day of that month. Medicines should not be disposed of via wastewater or household waste. Askyour pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
^6 FURTHER INFORMATION
What Nasobec Hayfever contains:
♦ The active ingredient is beclometasone dipropionate anhydrous.
♦ The other ingredients are phenylethyl alcohol, benzalkonium chloride, polysorbate 80, microcrystalline cellulose and carmellose sodium, glucose anhydrous and purified water. Your medicine may also contain hydrochloric acid to adjust the pH.
What Nasobec Hayfever looks like and contents of the pack:
♦ Nasobec Hayfever is a nasal spray.
♦ Nasobec Hayfever is supplied in polyethylene bottles in two pack sizes:
♦ 15 ml bottle containing 100 sprays.
♦ 30 ml bottle containing 180 sprays.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer Marketing authorisation holder:TEVA UK Limited, Eastbourne, BN22 9AG.
Company responsible for manufacture:TEVA Czech
Industries s.r.o., Opava, Czech Republic. _
This leaflet was last revised: March 2015
pL00289/1607 ^