Betagan 0.5% W/V Eye Drops Solution
5. How to store BETAGAN
Keep out of the reach and sight of children.
Do not use an unopened bottle of BETAGAN after the expiry date which is stated on the bottle label and the carton after "EXP’.’ The expiry date refers to the last day of that month.
Store BETAGAN in the original carton to protect it from light. Do not store above 25°C.
Do not use BETAGAN if you notice that the tamper-proof seal is broken. Once opened, solutions may become contaminated, which can cause eye infections. Therefore, you must throw away the bottle 4 weeks after you first opened it, even if some solution is left. To help you remember, write down the date that you opened it in the space on the carton.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.


6. Further information What BETAGAN contains
• The active substance is Levobunolol hydrochloride 0.5% w/v
• The other ingredients are poly(vinyl alcohol), benzalkonium chloride, sodium metabisulfite (E223), sodium chloride, disodium edetate, sodium phosphate dibasic heptahydrate, potassium dihydrogen phosphate, sodium hydroxide (to adjust pH) or hydrochloric acid (to adjust pH) and purified water
What BETAGAN looks like and contents of the pack
BETAGAN is a colourless to light yellow solution in a white plastic bottle.
Each pack contains 1 or 3 plastic bottles, each with a screw cap.
Each bottle contains 5 millilitres of solution and will be approximately half full.
Marketing Authorisation Holder
Allergan Limited Marlow International The Parkway Marlow
Bucks, SL7 1YL United Kingdom
Tel: 01628 494026
E-mail: uk_medinfo@allergan.com
Manufacturer
Allergan Pharmaceuticals Ireland
Castlebar Road
Westport
County Mayo
Ireland
Further information about glaucoma is available from:
International Glaucoma Association (IGA)
Tel: 01233 64 81 70 Fax: 01233 64 81 79 Email: info@iga.org.uk
(The IGA is an organisation which helps glaucoma patients and their relatives, and is not associated with Allergan.)
This leaflet was last revised in July 2014.
To listen to or request a copy of this leaflet in Braille, large print or audio please call, free of charge:
0800 198 5000 (UK only).
Please be ready to give the following information: Levobunolol 0.5% reference number PL 00426/0060.
This is a service provided by the Royal National Institute of Blind People.
Artwork created at 100%
Drop all keylines and notes before printing Part Number: 71995MD178F Drawing Number: 0106901
Read all of this leaflet carefully before you start using this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What BETAGAN is and what it is used for
2. What you need to know before you use BETAGAN
3. How to use BETAGAN
4. Possible side effects
5. How to store BETAGAN
6. Further information
1. What BETAGAN is and what it is used for
BETAGAN is an eye drop. It is used to treat glaucoma by lowering the pressure that builds up in the eye.
Your eyeball contains a watery liquid, which is constantly being drained out of the eye, and new liquid is made to replace this. Glaucoma can occur when the liquid does not drain out quickly enough. This leads to raised pressure within the eyeball which can eventually damage your sight. BETAGAN works by reducing the production of liquid. This reduces the pressure inside the eye.
BETAGAN belongs to a group of medicines called beta blockers.
2. What you need to know before you use BETAGAN Do not use BETAGAN if
• you are allergic to:
levobunolol benzalkonium chloride
any other ingredients (see section 6 "Further information”)
• you have or have had asthma or other lung diseases (for example severe chronic obstructive pulmonary disease (COPD)) where you have difficulty breathing, wheezing or a chronic cough
• you have or have had heart problems such as slow heart beat, heart failure or heart beat disorders (irregular heart beat).
BETAGAN is not recommended for use in children Take special care with BETAGAN
Talk to your doctor before using BETAGAN if you suffer from, or have in the past suffered from:
• coronary heart disease (symptoms can include chest pain or tightness, breathlessness or choking), heart failure, low blood pressure
• disturbances of heart rate such as slow heart beat
• asthma, breathing problems or chronic obstructive pulmonary disease
• poor blood circulation disease (such as Raynaud’s disease or Raynaud’s syndrome)
• diabetes as levobunolol may mask the signs and symptoms of low blood sugar
• overactivity of the thyroid gland as levobunolol may mask the signs and symptoms of this condition
• any allergies (especially if any of the allergies are severe)
• problems with the surface of your eyes
• eye surgery to lower the pressure in your eye(s)
If you have a history of breathing problems or heart disease your doctor may monitor you more closely as in rare cases death has been reported following taking levobunolol.
If you suffer from allergies, or have a severe allergic reaction be aware that the usual dose of adrenaline may need to be increased.
Tell your doctor before you have an operation that you are using BETAGAN as levobunolol may change effects of some medicines used during anaesthesia.
Other medicines and BETAGAN
Tell your doctor or pharmacist if you are using or have recently used any other medicines.
Tell your doctor before using BETAGAN if you are taking oral beta blockers to lower blood pressure.
BETAGAN is also a beta blocker. Therefore your doctor needs to know whether there is likely to be a risk of having too much beta blocker in your system. The following symptoms could result from such a risk:
• low blood pressure (for example, upon standing)
• slow heart beat
• dizziness/temporary loss of consciousness
• low pressure in the eye ball

It is important to tell your doctor before using BETAGAN, if you are taking:
• medicines to treat high blood pressure (hypertension)
• medicines for heart conditions (for example irregular heartbeat) such as beta blockers, amiodarone or digoxin
• another eye drop to lower pressure in the eye (glaucoma)
• medicines to dilate your pupil, for example adrenaline
• other beta blockers by mouth or as an eye drop
• medicines to treat diabetes

If the dose of any of your current medicines changes you should tell your doctor.
Pregnancy and breast-feeding
Tell your doctor before you start using BETAGAN if you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby.
BETAGAN should not be used during pregnancy or breast-feeding unless your doctor, having considered all the risks, still recommends it for you.
Ask your doctor or pharmacist for advice before using any medicine.
Driving and using machines
BETAGAN may cause tiredness, dizziness or blurred/abnormal vision. Do not drive or use machinery until these symptoms have cleared..............
Important information about some of the ingredients of BETAGAN
A preservative in BETAGAN (called benzalkonium chloride) may cause eye irritation and is also known to discolour soft contact lenses. Therefore, do not use the drops while your contact lenses are in your eyes. Wait at least 15 minutes after using the eye drops before putting your lenses back in your eyes.
BETAGAN contains sodium metabisulfite which may rarely cause allergic reactions, difficulty in breathing or wheezing.
3. How to use BETAGAN
Always use BETAGAN exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure. The usual dose is 1 drop into the affected eye(s), 1 to 2 times a day.
Your doctor will usually check your eye pressure 4 weeks after starting treatment. It may be necessary to use another drug with BETAGAN to control your condition.
Instructions for use
• You must not use the bottle if the tamper-proof seal on the bottle neck is broken before you first use it. Apply your eye drops in the following way:
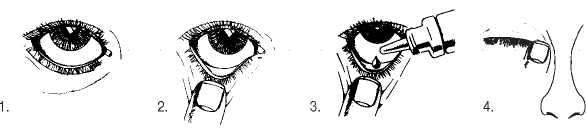
Wash your hands. Tilt your head back and look at the ceiling.
Gently pull the lower eyelid down until there is a small pocket.
Turn the bottle upside down and squeeze it to release one drop into each eye that needs treatment. Whilst keeping the treated eye closed, press your finger against its corner (where the eye meets the nose) and hold for two minutes.
1.
2.
3.
4.
If a drop misses your eye, try again.
To avoid contamination, do not let the tip of the dropper touch your eye or anything else.
Replace and tighten the cap straight after use.
Wipe off any excess liquid from your cheek with a clean tissue.
The proper application of your eye drops is very important. If you have any questions ask your doctor or pharmacist.
If you use more BETAGAN than you should
Putting too many drops in your eye(s) is unlikely to lead to unwanted side-effects. If you have placed too many drops in your eye(s), wash your eyes with clean water. Put your next dose in at the usual time.
If, by accident, anyone drinks this medicine, contact your doctor straight away.
If you forget to use BETAGAN
If you forget a dose apply it as soon as you remember, unless it is almost time for your next dose, in which case you should miss out the forgotten dose. Then apply your next dose as usual and continue with your normal routine.
Do not use a double dose to make up for a forgotten dose.
If you stop using BETAGAN
Do not stop using BETAGAN or reduce the amount you are using until your doctor tells you to, since your symptoms may get worse.
BETAGAN should be used as advised by your doctor. If you have any further questions on the use of this product, ask your doctor or pharmacist.
Like all medicines, BETAGAN can cause side effects, although not everyone gets them. The chance of having a side effect is described by the following categories:
|
Very common |
Occurs in more than 1 out of 10 patients |
|
Common |
Occurs in fewer than 1 out of 10 patients |
|
Uncommon |
Occurs in fewer than 1 out of 100 patients |
|
Rare |
Occurs in fewer than 1 out of 1,000 patients |
|
Very rare |
Occurs in fewer than 1 out of 10,000 patients |
|
Unknown |
The chance of having a side effect is unknown |
You should contact your doctor or go to a hospital immediately if you experience:
• rash, swallowing or breathing difficulties, swelling of your lips, face, throat or tongue (these could be signs of a serious allergic reaction)
• breathing difficulties (including asthma)
• loss of consciousness (or feeling like this)
• unusual or slow heart beat
• low blood pressure
The above side effects are serious and in rare cases can be life-threatening.
The following side effects may be seen with BETAGAN:
Very common side effects:
• eye irritation, eye pain
Common side effects:
• inflammation of the eyelids and outermost layer of the eye
Side effects with unknown frequency:
• eye redness, allergic reaction in the eye, decreased blinking, inflammation of the iris and inner eye or surface of the eye (cornea), blurring of vision, small breaks in the surface of the eye with inflammation, itching of the eye/eyelids, eye and/or eyelid swelling/puffiness, eye discharge or tearing, dry eye, redness and eczema of eyelids
• unusual, irregular, slow or a fast heart beat, fainting
• asthma, breathing difficulties, throat irritation, nasal discomfort
• low blood pressure, cold, numbness or discoloration of the hands or feet
• confusion, dizziness, sleepiness, decreased energy, headache, sleeping problems
• depression, nervousness
• swelling of the face, tiredness
• nausea
• skin rashes, hives, flaking and itching, rough patches of skin
Like other medicines applied into the eyes, levobunolol is absorbed into the blood. This may cause similar side effects as seen with beta-blocking agents taken by mouth or injected. Incidence of side effects after topical ophthalmic administration is lower than when medicines are taken by mouth or injected. The side effects listed below include additional reactions seen within the class of beta-blockers when used for treating eye conditions:
• Generalized allergic reactions including swelling beneath the skin that can occur in areas such as the face and limbs, and can obstruct the airway which may cause difficulty swallowing or breathing, severe sudden life-threatening allergic reaction.
• Stroke, reduced blood supply to the brain, increases in signs and symptoms of myasthenia gravis (muscle disorder) and unusual sensations like pins and needles.
• Low blood glucose levels.
• Nightmares and memory loss.
• Detachment of the layer below the retina that contains blood vessels following filtration surgery which may cause visual disturbances, corneal erosion (damage to the front layer of the eyeball), dropping of the upper eyelid (making the eye stay half closed), double vision.
• Heart attack, heart failure, chest pain, congestive heart failure (heart disease with shortness of breath and swelling of the feet and legs due to fluid build up), fluid build up in your limbs.
• Cold hands and feet.
• Constriction of the airways in the lungs (predominantly in patients with pre-existing diseases like
asthma), cough...............
• Taste disturbances, indigestions, diarrhoea, dry mouth, abdominal pain, vomiting.
• Hair loss, skin rash with white silvery coloured appearance (psoriasiform rash) or worsening of psoriasis.
• Muscle pain not caused by exercise.
• Sexual dysfunction, decreased libido.
Other side effects reported with eye drops containing phosphates:
In very rare cases, some patients with severe damage to the clear layer at the front of the eye (the cornea) have developed cloudy patches on the cornea due to calcium build-up during treatment.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via:
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information on the safety of this medicine.