Betaloc I.v. Injection
Read all of this leaflet carefully before you start medicine because it contains important informa
this
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or nurse.
• If you get any side effects, talk to your doctor or nurse.
I This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Betaloc IV is and what it is used for
2. What you need to know before you use Betaloc IV
3. How to use Betaloc IV
4. Possible side effects
5. How to store Betaloc IV
6. Contents of the pack and other information
Betaloc IV Injection contains a medicine called metoprolol tartrate. This belongs to a group of medicines called beta-blockers.
Betaloc IV Injection is used:
• To treat uneven heart beat (arrhythmia).
• After a heart attack.
It works by making your heart beat more slowly and with less force.
Do not have Betaloc IV Injection
• If you are allergic to metoprolol tartrate or any of the other ingredients of this medicine (listed in section 6).
you are allergic to any other beta-blocker medicines (such as atenolol or propanolol).
' you have ever had any of the following heart problems: heart attack with shock
heart failure which is not under control (this usually makes you breathless and causes your ankles to swell) second- or third-degree heart block (a condition which may be treated by a pacemaker)
very slow or very uneven heart beats (unless a permanent pacemaker is in place).
you have low blood pressure which may make you feel faint. you have very poor circulation.
you have atu
ia that is not being
treated. This is usually near your kidney and can cause high blood pressure. If you are being treated for phaeochromocytoma your doctor will give you another medicine called an alpha-blocker, to take as well as your Betaloc IV Injection.
“• TyoTiavi bTenl5ldlharyou¥avrhighi!r1ihan_normil levels of acid in your blood (metabolic acidosis).
If any of the above apply to you, do not have Betaloc IV Injection. If you are not sure talk to your doctor or nurse before having Betaloc IV Injection.
Warnings and precautions
Talk to your doctor or nurse before using Betaloc IV Injection
• If you have asthma, wheezing or any other similar breathing problems, or you get allergic reactions, for example to insect stings, foods or other substances. If you have ever had asthma or wheezing, do not have this medicine without first checking with your doctor.
• If you have a type of chest pain (angina) called Prinzmetal's angina.
• If you have poor blood circulation or controlled heart failure.
• If you have first-degree heart block.
• If you have problems with your liver.
• If you have diabetes. Your treatment for diabetes may need to be adjusted.
• If you have thyrotoxcosis (a condition caused by an overactive thyroid gland). Your medicine may hide the symptoms of
• If you have or have ever had psoriasis (a skin condition).
If you are not sure if any of the above apply to you, talk to your doctor before having Betaloc IV Injection.
Other medicines and Betaloc IV Injection Tell your doctor or nurse if you are taking, have recently taken or might take any other medicines. This includes medicines that you buy without a prescription and herbal medicines. This is because Betaloc IV Injection can affect the way some other medicines work and some medicines can have an effect on Betaloc V Injection.
In particular, tell your doctor or nurse if you are taking any of the following medicines:
• Clonidine (for high blood pressure or migraine). If you are taking clonidine and Betaloc V Injection together, do not stop taking clonidine unless your doctor tells you to do so. If you have to stop taking clonidine or Betaloc IV Injection, your doctor will give you careful instructions about how to do it.
• Medicines called Mono-Amine Oxidase Inhibitors (MAOIs).
• Verapamil, diltiazem or nifedipine (for high blood pressure or chest pain).
• Quinidine, amiodarone or digoxin (for heart problems).
• Hydralazine (for high blood pressure).
• Medicines for stomach ulcers (such as cimetidine).
• Medicines for infections caused by rilfampicin).
• Adrenaline, also known as epinephrine (a medicine that stimulates the heart).
• Medicines for pain, inflammation and arthritis (such as indometacin and celecoxib).
• Medicines for depression.
• Medicines for mental illness (such as phenothiazine).
• Barbiturates (a type of sedative).
• Anti-histamines (medicines for hay fever and allergies).
• Other beta-blocker medicines used as eye drops (such as timolol).
• Insulin or medicines that you take by mouth for diabetes.
Your doctor may need to adjust your dose of these medicines.
• Lidocaine (a local anaesthetic).
• Ergotamine medicines (for migraines).
Operations
If you go into hospital or to the dentist to have an operation,
tell the anaesthetist, medical staff or dentist that you are using
Betaloc IV Injection. This is because you can get low blood pressure (hypotension) if you are given certain anaesthetics
while you are taking Betaloc IV Injection.
Betaloc IV Injection with food , drink and alcohol
Before having Betaloc IV Injection, inform your doctor or nurse if you have recently had alcohol. This is because alcohol can affect how the medicine works.
Pregnancy, breast-feeding and fertility
• If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or nurse for advice before taking this medicine. Beta-blockers including Betaloc IV Injection may cause harm to the foetus and early labour.
• If you become pregnant while having Betaloc IV Injection, talk to your doctor as soon as possible.
Driving and using machines
If you feel dizzy o r tired after using this medicine, do not drive or use any tools or machines.
Adults
Betaloc IV Injection will be given to you by a doctor or a nurse. It will be given to you as an injection into your vein. Your doctor will decide how much to give you. The amount depends on your illness. Children
Your medicine must not be given to children.
Please turn over ^
I. Name of the Medicinal Product
' eshis of "*■* p1 Eur
3: pszt, jr
4:' iSf Sir,,, supraventricular ta.hparrhpth.ia,
Ei'prii;::,T,^a:tSi^o:JlVii^r”l”v^i:tr'.•u^ zesrP“s
may also decrease the need for opiate analgesics.

V Injection or other
er beta-blockers should net be used in
*, or t” any of the ^^115
vn to reduce mortality when administered
s be adjusted to the individual requirements of the

,Duu=“^
i"H:;i'i:^S^ri;:l^ius^”d5””;;^t;",l ;r;;^i",“„dSiqu;id . a
Myocardial infarction: Intravenous Betaloc I.V. Injection should be initiated
^mtp.•rs;^5r”u;^;ird;.D”;sis■di;“t”;!3:r^"li;,did,til„ ^i^ddu:t•o^i;^1d”l.,^wi■,irbi' e."1 s” t’,“ ■
D”i”ir■djt"s.mi^;llSi:eS*^•idid ””,"dir,p1•i”!“Uut^•’■ri“,d™S"■tl”. »
p..di.,„a _„ul.,,on: ,f.y ■ uB ” ...p— „ .BB™ 1,s
srVsXe.-t; E”■d;;l,^dd^■*id/l^:)sfUrS!•d?gd,.■li•■.mIsrd”l■l
systolic blood pressure <100 mmHg and'er severe heart failure.


• ipfg
• In labile and msulindependent diabetes
• tyde
J M—
■!iSrseEU!iitbi^i”iiU.dii!iCS
to patients diabetes it may be necessary to adjust
of calcium antagensts of the verapamil-type should net be given.
BifSr,^yyl•::■iCiBt^f■tq•ilr„r”!ypi^■d„;i■•X■d”d„;)”t"d" s;s•B?.yu^slXu™r■;B•:,1■;i;■Bul;:d^i■r5^■■■Sl■"i■si
asas^^
B^■XplVSBO^id"l■::i^'la:;?s;:i5^c1i,.'i:I bresoi:,;,;;: essi .ii"
Meteprelel may impair the elimination of Moraine
TXss eier■„ts■;“pl•i.^pL■^rs■5e ef insulin ~y te b
Please turn over
effect on blood pressure are usually additive. Care should be taken when
EHtnefite;; cojjoivntcontrofoi'hhsepeeesvdrugs 'f,'n te L“^d ~*h
BeScc IV Injection should not be used in pregnancy or nursing mothers

ara!;tff,bhocfe5vB?ogoCca.,mnJectt^:m;yuSaas5^nheh.•' ::h;;nn:inTaherSaa:;Tl^ea„sJehtSkhOf»cer;~atvfnhbE:nm ;sse^L;fa^^5.hE£sotrtfyKnE^n;her.cSs;e.n‘Le; p;fnEe;,afte; ~anSknn.gp;s^f;n;ln,n
cord blood, no evidence of foetal abnormalities has been reported. Breast-feeding
Breast-feeding is not recommended. The amount of metoprolol ingested
neons rmsd ;otreparoeddu~:ths,<n:rf:aantth5aapebr;ingsSectsin tle
4.7 Effects on ability to drive and use machines
se;ce:;;ti|:n''Hlo!:even: h^fne;;ndte^Lkent;n,o^acc^,snll«f!',hts:n;nnfflSse
dizziness or fatigue may occur.
I The following events have been reported as adverse events in clinical trials
or reported from routine use.
J.1e,f;^:;nagnd(f,is;oni:fa;rsu,;n:iies,^:anaa..an
| <1/100), rare ((>1/10,000 la <1/1,000) and very rare (<1/10,000).
MeChanism, “ti. beta-adrenoceptor bt beta-ahrenoteptors _ (conferring some
adrenoceptor blocking actvity comparable in potency with propranolol.
fcna:seaeeoa.tpS, aco^s ircf
maptdpyroiocrease following acute ac ChleOISen'tfClnd:oCyreahtatSf•c,yMMIT included 45,852 patients admitted to
^o;ncp^a,';d~th;inair4t,:;c;ur.Sth,Vh,JpCrlse,)dEfcSym,pbc;:;a01,,n'e:n^!;,^t•s•T';ttLt,'afo n
ST depression or left bundle-branch block). Patients were randomly
............ ' ,^l~etehesnl20ec,:^pdita,T-^)^rt~c
.. hsiran?
,er of the co-primary outcomes was significantly reduced by metopr,
fedwreatrepoefo
If you use more Betaloc IV Injection than you should
If you think you have been given too much of this medicine, talk to your doctor or nurse straight away.
If you stop using Betaloc IV Injection
Your doctor or nurse will let you know when to stop having this medicine. You may need to stop having it gradually.
If you have any further questions on the use of this medicine, ask your doctor or nurse.
Class Frequency _

Tndesiiable EllgCt
Excess frequency of 0.4 % compared ~th placebo in a study of Reporting of suspected adverse reactions
sess rjsilsSE ;efcrnlngfSer-aL^h^;eff,ti;nk^;,Jhe,t:E.hcinfn^;ro;tu^r p,ra5S.T.■SlSn,;nlsh.s;e1h£/^fE^^
nocanntendc~elteee~lrep:oEaerdh:;e'nd. Snhohaemc5y^aeecfarn; dnsta:eer afhsnn:cT^;^■;Eas■aScnLirnfainna ^en^ard
those who were stable, particularly after days 0-1
■2 BhSlahsnOmOa^lonProPel1ie*
Met0p0ot,:|0^n eliminated mainly by hepatic metabolism, the average elimination half-life is 3.5 hours (range 1-9 hours). Rates of metabolism vary between
co;nc,EnLElSlc:^nhinocn^c;~eaboEsea;Cf;rph;n,5,fts,;.mJeb~Srh^h.ehS^•nma
5.3 Preclinical safety data
metoprolol tartrate has been established after many years of clinical use.
icentration impairment, somn-“sia/memory impairment, ta
^ 'h1*3181
bancesof cardac conduction,.
Postural hlnorhern (very rarely with
nte
Symptoms^ ^ I de hy t d ft
Atropine, adrenostimulating drugs ' or pacemaker to treat bradycardia and Hypotension, acute cardiac failure, and shock to be treated with suitable volume
fiSffsd:ng:Lc^iJn^flnl;f£:g;nLnia3;?ten:na^in';;^'f;hhi^n,;:;£a;;;)nh^Lugn
suah asdabulamine, wrth a, receptor agonistic drugs added in presence of vasodilation. Intravenous use o Ca2* can also be considered.
Bronchospasm can usually be reversed by bronchohllators.
Sodium chloride and water for injections.
6,2 ss,eies
6.4 Special precautions for storage Protect from lght. Store below 25 °C.
6.5 Nature and contents of container 5 ml glass ampoule.
6.6 Special precautions for disposal
7. Marketing Authorisation Holder
AstraZeneca UK Limited,
600 Capability Green,
Luton, LU1 3LU, UK.
8. Marketing Authorisation Number PL 17901/0106
Leaflet updated: May 2014
5. Pharmacological Properties
Phamacotherapeutic group: Beta blocking agents, selective ATC code: C07AB02
© AstraZeneca 2014 CV 14 0052
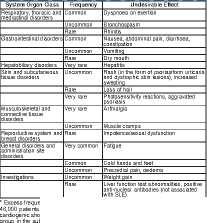
Like all medicines, this medicine can cause side effects, although not everybody gets them. The following side effects may happen with this medicine.
Very common (may affect more than 1 in 10 people)
• Feeling tired.
Common (may affect up to 1 in 10 people)
• You may notice that your pulse rate becomes slower while you are having Betaloc V Iniecton. If this happens tell your doctor as soon as possible. Your doctor may need to lower your dose of Betaloc V Injection or you may need to stop having it gradually.
• Pounding heart beat.
• Dizziness (particularly when standing up, may sometimes cause fainting).
• Headache.
• Shortness of breath on effort.
• Feeling sick (nausea).
• Stomach ache.
• Diarrhoea or constipation.
• Cold hands and feet.
Tncommon (may affect up to 1 in 100 people).
• Depression.
• Difficulty going to sleep.
• Nightmares.
• Difficulty concentrating.
• Feeling sleepy.
• Sensation of burning, prickling or numbness.
• Heart changes shown on an ECG.
• Severe drop in blood pressure during a heart attack (cardiogenic shock)
• Feeling of tightness in the airways.
• Being sick (vomiting).
• Skin rash.
• Increased sweating.
• Muscle cramps.
• Chest pain.
• Swelling.
• Weight gain.
Rare (may affect up to 1 in 1,000 people)
• Feeling anxious or nervous.
• Disturbances of vision.
• Dry or irritated eyes.
• Uneven heart beat.
• Numbness and spasm in your fingers (Raynaud's disease).
• Allergic reactions. The signs may include runny nose and red or watery eyes.
• Dry mouth.
• Thinning of your hair.
• Being unable to get an erection (impotence).
• Liver problems (shown in a blood test).
Very rare (may affect up to 1 in 10,000 people)
• Changes to some of the cells or other parts of your blood. Your doctor may take blood samples every so often to check whether Betaloc IV Injection has had any effect on your blood.
• Reduced numbers of platelets in the blood. This may make you bruise more easily.
Confusion.
Hallucinati ons.
Loss of memory or problems with memory.
Changes to taste.
Ringing in the ears.
Inflammation of the liver (hepatitis).
Skin reaction due to increased sensitivity to sunlight.
Pain in joints.
If you have any of the following conditions, they may get worse when you start to take your medicine:
• Being short of breath, feeling tired or having swollen ankles (if you have heart failure) may get worse for a while. This is uncommon affecting less than 1 in 100 people.
• Psoriasis (a skin condition) and poor circulation may get worse. This is very rare affecting less than 1 in 10,000 people.
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme. Website: www.mhra.gov.uk/yellowcard.
• The doctor and hospital pharmacist are responsible for storing, using and disposing of Betaloc IV Injection correctly.
• Keep this medicine out of the sight and reach of children.
• Do not store above 25°C. Store your medicine where it is protected from light.
• This medicine should be used immediately after opening.
• Do not use your medicine after the expiry date which is stated on the carton. The expiry date refers to the last day of that month.
What Betaloc IV Injection contains
The active substance is metoprolol tartrate. Each 5 ml (millilitre) ampoule contains 5 mg (milligrams) of metoprolol tartrate (this is equal to 1 mg of metoprolol tartrate per ml).
The other ingredients are sodium chloride and water for iniectons. What Betaloc IV Injection looks like and contents of the pack Betaloc IV Injection comes in ampoules. Each ampoule contains 5 ml of a clear colourless liquid.
Marketing Authorisation Holder and Manufacturer
The Marketing Authorisation for Betaloc IV Injection is held by AstraZeneca UK Limited, 600 Capability Green, Luton, LU1 3LU UK. Betaloc IV Injection is manufactured by CENEXI, 52 rue Marcel et Jacques Gaucher, 94120 FONTENAY-SOUS-BOIS, France.
To listen to or request a copy of this leaflet in Braille, large print or audio please call, free of charge:
0800 198 5000 (UK only)
Please be ready to give the following information:
Product name Betaloc IV Injection
Reference number 17901/0106 This is a service provided by the Royal National Institute of Blind People.
Leaflet prepared: May 2014.
© AstraZeneca 2014.
Betaloc is a trade mark of the AstraZeneca group of companies. CV 14 0052
By reporting side effects you can help provide more information on the safety of this medicine.