Betoptic 0.25% W/V Eye Drops Suspension
Using other medicines
Using other medicines
S307 LEAFLET Betoptic 20150619
PACKAGE LEAFLET: INFORMATION FOR THE USER BETOPTIC® 0.25% w/v EYE DROPS SUSPENSION (betaxolol hydrochloride)
Your medicine is marketed using the above name but will be
referred to as Betoptic Eye Drops throughout the following patient
information leaflet.
Read all of this leaflet carefully before you start using this
medicine
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or your pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
IN THIS LEAFLET
1. What Betoptic Eye Drops are and what they are used for
2. Before you use Betoptic Eye Drops
3. How to use Betoptic Eye Drops
4. Possible side effects
5. How to store Betoptic Eye Drops
6. Further information
1. WHAT BETOPTIC EYE DROPS ARE AND WHAT THEY ARE USED FOR
Betoptic Eye Drops belongs to a group of medicines known as beta blockers.
It is used to treat chronic open-angle glaucoma or ocular hypertension (high pressure in the eye) by reducing the fluid pressure in your eye (s).
2. BEFORE YOU USE BETOPTIC EYE DROPS
Do not use Betoptic Eye Drops, suspension...
• If you are allergic to betaxolol, beta-blockers or any of the other ingredients listed in section 6.
• If you have now or have had in the past, respiratory problems such as severe asthma, severe chronic obstructive bronchitis (severe lung condition which may cause wheeziness, difficulty in breathing and/or long-standing cough).
• If you have a slow heart beat, heart failure or disorders of heart rhythm (irregular heart beats).
Ask your doctor for advice.
Take special care...
Before you use this medicine, tell your doctor if you have now or
have had in the past
• coronary heart disease (symptoms can include chest pain or tightness, breathlessness or choking), heart failure, low blood pressure (hypotension)
• disturbances of heart rate such as slow heart beat (bradycardia)
• breathing problems, asthma or chronic obstructive pulmonary disease (lung disease which may cause wheeziness, difficulty in breathing and/or long-standing cough).
• poor blood circulation disease (such as Raynaud's disease or Raynaud's syndrome)
• diabetes, as betaxolol may mask the signs and symptoms of low blood sugar
• overactivity of the thyroid gland as betaxolol may mask the signs and symptoms
• angle-closure glaucoma
• dry eyes (Sicca Syndrome)
Tell your doctor before you have an operation that you are using
Betoptic Eye Drops as betaxolol may change the effects of some
medicines used during anaesthesia.
If any of these apply you may still be able to use Betoptic Eye
Drops, but discuss it with your doctor first.
Betoptic Eye Drops can affect or be affected by other medicines you are using including other eye drops for the treatment of glaucoma. Tell your doctor if you are using or intend to use medicines to lower blood pressure, heart medicine, medicines to treat diabetes or medicines to treat emotional, behavioural or mental disorders such as anxiety or depression.
Betoptic Eye Drops may reduce the effectiveness of adrenaline, which can be used to treat serious allergic reactions (anaphylaxis). Tell your doctor if you have a history of anaphylaxis or allergic reactions.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
If you are using more than one type of eye drop, wait 5 minutes between using each one. Eye ointments should be administered last.
Pregnancy and breast-feeding
Do not use Betoptic Eye Drops if you are pregnant unless your doctor considers it necessary.
Do not use Betoptic Eye Drops if you are breast-feeding.
Betaxolol may get into your breast-milk.
Ask your doctor for advice before taking any medicine during breast-feeding.
Driving and using machines
If your sight is affected in any way following the use of Betoptic Eye Drops, you should not drive or use any machines.
Important information if you wear Contact Lenses
Do not use the drops while wearing contact lenses. Wait at least 15 minutes after use before putting your lenses back in. There is a preservative in Betoptic Eye Drops (benzalkonium chloride) that can discolour soft contact lenses.
3. HOW TO USE BETOPTIC EYE DROPS
Betoptic Eye Drops should only be used in the eye(s)
The usual dose
The usual dose is 1 drop in the affected eye(s) twice daily.
Remove the loose collar from the cap when you open the bottle. Not recommended for use in CHILDREN
Always use Betoptic Eye Drops exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
How to use
• Wash your hands before you start.
• Shake the bottle well.
• Twist off the bottle cap.
• Hold the bottle pointing down, between your thumb and fingers.
• Tilt your head back.
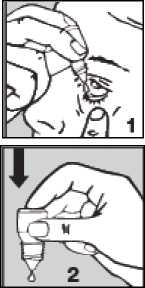
• Pull down your lower eyelid with a finger, until there is a ‘pocket' between the eyelid and your eye.
The drop will go in here (picture 1).
• Bring the bottle tip close to the eye.
Do this in front of a mirror if it helps.
• Do not touch your eye or eyelid,
surrounding areas or other surfaces with the tip of the vial. It could infect the drops.
• Gently press on the base of the
bottle to release one drop at a time (picture 2).
• Do not squeeze the bottle, only a gentle press on the bottom is needed.
• If you use drops in both eyes, repeat the steps for your other eye. Put the bottle cap firmly back on immediately after use.
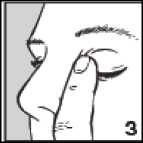
• After using Betoptic Eye Drops, press a finger into the corner of your eye by the nose (picture 3) for 2 minutes. This helps to stop betaxolol getting into the rest of the body.
• If a drop misses your eye, try again.
• If you miss a dose, just take it as soon as possible. However, if it is almost time for your next dose, skip the missed dose and go back to your regular dosing schedule. Do not use a double dose to make up for a missed dose.
* If you use more Betoptic Eye Drops than you should it can
be washed out of your eye with warm water.
If you have any further questions on the use of Betoptic Eye Drops, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Betoptic Eye Drops suspension can cause side effects, although not everybody gets them.
You can usually carry on taking the drops, unless the effects are serious. If you are worried, talk to a doctor or pharmacist. Do not stop using Betoptic Eye Drops without speaking to your doctor.
Like other medicines applied into eyes, betaxolol is absorbed into the blood. This may cause similar side effects as seen with beta-blockers given by injection or taken by mouth. Incidence of side effects after beta-blockers are used in the treatment of eye conditions is lower than when medicines are, for example, taken by mouth or injected. Listed side effects include reactions seen following treatment with Betaxolol eye drops and within the class of other beta-blockers used for treating eye conditions.
Side effects experienced by patients during clinical trials with BETAXOLOL eye drops are:
Very common: may affect more than 1 in 10 users
* Eye discomfort (includes a feeling of something in the eye)
Common: (affects 1 to 10 users in 100)
* Blurred vision, watery eyes
* Headache
Uncommon (affects 1 to 10 users in 1000):
* Inflammation of the eye surface, conjunctivitis or symptoms of conjunctivitis, visual impairment, sensitivity to light, painful, dry or tired eyes, excessive blinking, irritated, red or swollen eyes, a feeling of something in the eye, eye itchiness, eye discharge, weeping eyelids, bloodshot eyes.
* Slow heart beat or unusually rapid heart beat
* Asthma, difficulty breathing, blocked nose
* Nausea
Rare (affects 1 to 10 users in 10,000):
* Cataract formation, decreased sensitivity of the eye, inflammation of the eyelid
* Anxiety, difficulty sleeping (insomnia), depression
* Fainting
* Low blood pressure
* Cough, runny nose
* Taste disturbances
* Inflamed, itchy skin or rash, hair loss
* Libido decreased
The following side effects have also been reported by people using BETAXOLOL eye drops. The frequency cannot be estimated from the available data:
* Hypersensitivity reaction
* Dizziness
* Changes in the rhythm or speed of the heartbeat
* Loss or lack of strength
Additional side effects have been seen with other ophthalmic beta blockers and could potentially occur with Betoptic Eye Drops. The frequency is unknown.
* Generalised allergic reactions including swelling beneath the skin (that can occur in areas such as the face and limbs, and can obstruct the airway which may cause difficulty swallowing or breathing), hives (or itchy rash), localised and generalised rash, itchiness, severe sudden life-threatening allergic reaction.
* Low blood glucose levels.
* Nightmares, memory loss, hallucinations, delusions and confusion.
* Stroke, reduced blood supply to the brain, increases in signs and symptoms of myasthenia gravis (muscle disorder), unusual sensations (like pins and needles).
* Detachment of the layer below the retina that contains blood vessels following filtration surgery which may cause visual disturbances, corneal erosion (damage to the front layer of the
* Chest pain, palpitations, oedema (fluid build up), congestive heart failure (heart disease with shortness of breath and swelling of the feet and legs due to fluid build up), a type of heart rhythm disorder, heart attack, heart failure.
* Raynaud's phenomenon, cold hands and feet with a blue colour, leg pains (especially if you have a history of poor circulation).
* Constriction of the airways in the lungs (predominantly in patients with pre-existing disease).
* Indigestion, diarrhoea, dry mouth, abdominal pain, vomiting.
* Skin rash with white silvery coloured appearance (psoriasiform rash) or worsening of psoriasis.
* Muscle pain not caused by exercise.
* Sexual dysfunction, impotence.
* Tiredness.
An increase in anti-nuclear antibodies has also been seen in patients taking ophthalmic beta-blockers
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE BETOPTIC EYE DROPS
* KEEP OUT OF THE SIGHT AND REACH OF CHILDREN.
* Do not store above 25°C.
* Store the bottle in the outer carton in order to protect from light.
* Stop using the bottle 28 Days after first opening, to prevent infections.
* Do not use after the expiry date printed on the carton label or bottle.
* If the medicine becomes discoloured or show any other signs of deterioration, you should seek the advice of your pharmacist who will tell you what to do.
* Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment
6. FURTHER INFORMATION
What Betoptic Eye Drops contain
* The suspension contains the active ingredient betaxolol hydrochloride equivalent to 0.25%w/v betaxolol base.
* Betoptic Eye Drops also contain the following: carbomer, boric acid, mannitol, N-lauroylsarcosine, benzalkonium chloride, poly (styrene divinylbenzene) sulfonic acid, disodium edetate and purified water. It may also contain small amounts of hydrochloric acid and/or sodium hydroxide to adjust the acidity or alkalinity of the product to ensure comfort of the product in the eye.
What Betoptic Eye Drops look like and contents of the pack
Betoptic Eye Drops are packaged in a carton containing a 5ml plastic bottle fitted with a dropper plug, white plastic screw cap and tamper evident ring. The bottle contains a cloudy white suspension.
Betoptic Eye Drops are available in bottles containing 5ml of solution.
Product Licence holder
Procured from within the EU and repackaged by the Product Licence holder: S&M Medical Ltd, Chemilines House,
Alperton Lane, Wembley, HA0 1Dx.
Manufacturer
This product is manufactured by either of the below manufacturers* ALCON CUSI S.A., Camil Fabra 58, 08320 El Masnou, Barcelona, Spain.
| POM | PL: 19488/0307 Leaflet revision date: 19 June 2015
Betoptic is a registered trade mark of Alcon Inc, Switzerland.
S307 LEAFLET Betoptic 20150619
S307 LEAFLET Betaxolol 20150619
PACKAGE LEAFLET: INFORMATION FOR THE USER BETAXOLOL HYDROCHLORIDE 0.25% w/v EYE DROPS SUSPENSION
Your medicine is marketed using the above name but will be referred to as Betaxolol Eye Drops throughout the following patient information leaflet.
Read all of this leaflet carefully before you start using this medicine
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or your pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
IN THIS LEAFLET
1. What Betaxolol Eye Drops are and what they are used for
2. Before you use Betaxolol Eye Drops
3. How to use Betaxolol Eye Drops
4. Possible side effects
5. How to store Betaxolol Eye Drops
6. Further information
1. WHAT BETAXOLOL EYE DROPS ARE AND WHAT THEY ARE USED FOR
Betaxolol Eye Drops belongs to a group of medicines known as beta blockers.
It is used to treat chronic open-angle glaucoma or ocular hypertension (high pressure in the eye) by reducing the fluid pressure in your eye (s).
2. BEFORE YOU USE BETAXOLOL EYE DROPS
Do not use Betaxolol Eye Drops, suspension...
• If you are allergic to betaxolol, beta-blockers or any of the other ingredients listed in section 6.
• If you have now or have had in the past, respiratory problems such as severe asthma, severe chronic obstructive bronchitis (severe lung condition which may cause wheeziness, difficulty in breathing and/or long-standing cough).
• If you have a slow heart beat, heart failure or disorders of heart rhythm (irregular heart beats).
Ask your doctor for advice.
Take special care...
Before you use this medicine, tell your doctor if you have now or
have had in the past
• coronary heart disease (symptoms can include chest pain or tightness, breathlessness or choking), heart failure, low blood pressure (hypotension)
• disturbances of heart rate such as slow heart beat (bradycardia)
• breathing problems, asthma or chronic obstructive pulmonary disease (lung disease which may cause wheeziness, difficulty in breathing and/or long-standing cough).
• poor blood circulation disease (such as Raynaud's disease or Raynaud's syndrome)
• diabetes, as betaxolol may mask the signs and symptoms of low blood sugar
• overactivity of the thyroid gland as betaxolol may mask the signs and symptoms
• angle-closure glaucoma
• dry eyes (Sicca Syndrome)
Tell your doctor before you have an operation that you are using
Betaxolol Eye Drops as betaxolol may change the effects of some
medicines used during anaesthesia.
If any of these apply you may still be able to use Betaxolol Eye
Drops, but discuss it with your doctor first.
Betaxolol Eye Drops can affect or be affected by other medicines you are using including other eye drops for the treatment of glaucoma. Tell your doctor if you are using or intend to use medicines to lower blood pressure, heart medicine, medicines to treat diabetes or medicines to treat emotional, behavioural or mental disorders such as anxiety or depression.
Betaxolol Eye Drops may reduce the effectiveness of adrenaline, which can be used to treat serious allergic reactions (anaphylaxis). Tell your doctor if you have a history of anaphylaxis or allergic reactions.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
If you are using more than one type of eye drop, wait 5 minutes between using each one. Eye ointments should be administered last.
Pregnancy and breast-feeding
Do not use Betaxolol Eye Drops if you are pregnant unless your doctor considers it necessary.
Do not use Betaxolol Eye Drops if you are breast-feeding. Betaxolol may get into your breast-milk.
Ask your doctor for advice before taking any medicine during breast-feeding.
Driving and using machines
If your sight is affected in any way following the use of Betaxolol Eye Drops, you should not drive or use any machines.
Important information if you wear Contact Lenses
Do not use the drops while wearing contact lenses. Wait at least 15 minutes after use before putting your lenses back in. There is a preservative in Betaxolol Eye Drops (benzalkonium chloride) that can discolour soft contact lenses.
3. HOW TO USE BETAXOLOL EYE DROPS
Betaxolol Eye Drops should only be used in the eye(s)
The usual dose
The usual dose is 1 drop in the affected eye(s) twice daily.
Remove the loose collar from the cap when you open the bottle.
Not recommended for use in CHILDREN
Always use Betaxolol Eye Drops exactly as your doctor has told
you. You should check with your doctor or pharmacist if you are not
sure.
How to use
• Wash your hands before you start.
• Shake the bottle well.
• Twist off the bottle cap.
• Hold the bottle pointing down, between your thumb and fingers.
• Tilt your head back.
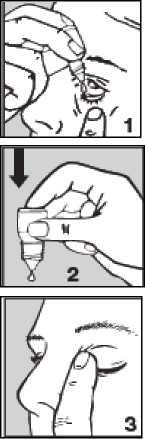
• Pull down your lower eyelid with a finger, until there is a ‘pocket' between the eyelid and your eye. The drop will go in here (picture 1).
• Bring the bottle tip close to the eye.
Do this in front of a mirror if it helps.
• Do not touch your eye or eyelid,
surrounding areas or other surfaces with the tip of the vial. It could infect the drops.
• Gently press on the base of the
bottle to release one drop at a time (picture 2).
• Do not squeeze the bottle, only a gentle press on the bottom is needed.
• If you use drops in both eyes, repeat the steps for your other eye. Put the bottle cap firmly back on immediately after use.
• After using Betaxolol Eye Drops, press a finger into the corner of your eye by the nose (picture 3) for 2 minutes. This helps to stop betaxolol getting into the rest of the body.
• If a drop misses your eye, try again.
• If you miss a dose, just take it as soon as possible. However, if it is almost time for your next dose, skip the missed dose and go back to your regular dosing schedule. Do not use a double dose to make up for a missed dose.
* If you use more Betaxolol Eye Drops than you should it can
be washed out of your eye with warm water.
If you have any further questions on the use of Betaxolol Eye Drops, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Betaxolol Eye Drops suspension can cause side effects, although not everybody gets them.
You can usually carry on taking the drops, unless the effects are serious. If you are worried, talk to a doctor or pharmacist. Do not stop using Betaxolol Eye Drops without speaking to your doctor.
Like other medicines applied into eyes, betaxolol is absorbed into the blood. This may cause similar side effects as seen with beta-blockers given by injection or taken by mouth. Incidence of side effects after beta-blockers are used in the treatment of eye conditions is lower than when medicines are, for example, taken by mouth or injected. Listed side effects include reactions seen following treatment with Betaxolol Eye Drops and within the class of other beta-blockers used for treating eye conditions.
Side effects experienced by patients during clinical trials with Betaxolol Eye Drops are:
Very common: may affect more than 1 in 10 users
* Eye discomfort (includes a feeling of something in the eye)
Common: (affects 1 to 10 users in 100)
* Blurred vision, watery eyes
* Headache
Uncommon (affects 1 to 10 users in 1000):
* Inflammation of the eye surface, conjunctivitis or symptoms of conjunctivitis, visual impairment, sensitivity to light, painful, dry or tired eyes, excessive blinking, irritated, red or swollen eyes, a feeling of something in the eye, eye itchiness, eye discharge, weeping eyelids, bloodshot eyes.
* Slow heart beat or unusually rapid heart beat
* Asthma, difficulty breathing, blocked nose
* Nausea
Rare (affects 1 to 10 users in 10,000):
* Cataract formation, decreased sensitivity of the eye, inflammation of the eyelid
* Anxiety, difficulty sleeping (insomnia), depression
* Fainting
* Low blood pressure
* Cough, runny nose
* Taste disturbances
* Inflamed, itchy skin or rash, hair loss
* Libido decreased
The following side effects have also been reported by people using Betaxolol Eye Drops. The frequency cannot be estimated from the available data:
* Hypersensitivity reaction
* Dizziness
* Changes in the rhythm or speed of the heartbeat
* Loss or lack of strength
Additional side effects have been seen with other ophthalmic beta blockers and could potentially occur with Betaxolol Eye Drops. The frequency is unknown.
* Generalised allergic reactions including swelling beneath the skin (that can occur in areas such as the face and limbs, and can obstruct the airway which may cause difficulty swallowing or breathing), hives (or itchy rash), localised and generalised rash, itchiness, severe sudden life-threatening allergic reaction.
* Low blood glucose levels.
* Nightmares, memory loss, hallucinations, delusions and confusion.
* Stroke, reduced blood supply to the brain, increases in signs and symptoms of myasthenia gravis (muscle disorder), unusual sensations (like pins and needles).
* Detachment of the layer below the retina that contains blood vessels following filtration surgery which may cause visual disturbances, corneal erosion (damage to the front layer of the
* Chest pain, palpitations, oedema (fluid build up), congestive heart failure (heart disease with shortness of breath and swelling of the feet and legs due to fluid build up), a type of heart rhythm disorder, heart attack, heart failure.
* Raynaud's phenomenon, cold hands and feet with a blue colour, leg pains (especially if you have a history of poor circulation).
* Constriction of the airways in the lungs (predominantly in patients with pre-existing disease).
* Indigestion, diarrhoea, dry mouth, abdominal pain, vomiting.
* Skin rash with white silvery coloured appearance (psoriasiform rash) or worsening of psoriasis.
* Muscle pain not caused by exercise.
* Sexual dysfunction, impotence.
* Tiredness.
An increase in anti-nuclear antibodies has also been seen in patients taking ophthalmic beta-blockers
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE BETAXOLOL EYE DROPS
* KEEP OUT OF THE SIGHT AND REACH OF CHILDREN.
* Do not store above 25°C.
* Store the bottle in the outer carton in order to protect from light.
* Stop using the bottle 28 Days after first opening, to prevent infections.
* Do not use after the expiry date printed on the carton label or bottle.
* If the medicine becomes discoloured or show any other signs of deterioration, you should seek the advice of your pharmacist who will tell you what to do.
* Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment
6. FURTHER INFORMATION
What Betaxolol Eye Drops contain
* The suspension contains the active ingredient betaxolol hydrochloride equivalent to 0.25%w/v betaxolol base.
* Betaxolol Eye Drops also contain the following: carbomer, boric acid, mannitol, N-lauroylsarcosine, benzalkonium chloride, poly (styrene divinylbenzene) sulfonic acid, disodium edetate and purified water. It may also contain small amounts of hydrochloric acid and/or sodium hydroxide to adjust the acidity or alkalinity of the product to ensure comfort of the product in the eye.
What Betaxolol Eye Drops look like and contents of the pack
Betaxolol Eye Drops are packaged in a carton containing a 5ml plastic bottle fitted with a dropper plug, white plastic screw cap and tamper evident ring. The bottle contains a cloudy white suspension.
Betaxolol Eye Drops are available in bottles containing 5ml of solution.
Product Licence holder
Procured from within the EU and repackaged by the Product Licence holder: S&M Medical Ltd, Chemilines House,
Alperton Lane, Wembley, HA0 1Dx.
Manufacturer
This product is manufactured by either of the below manufacturers* ALCON CUSI S.A., Camil Fabra 58, 08320 El Masnou, Barcelona, Spain.
| POM | PL: 19488/0307 Leaflet revision date: 19 June 2015
S307 LEAFLET Betaxolol 20150619