Biphozyl Solution For Haemodialysis / Haemofiltration
Biphozyl
UK, IE, MT Package leaflet: Information for the user.............................3
HU Betegtajekoztato: Informaciok a felhasznalo szamara........6
RO Prospect: Informal pentru utilizator....................................9
BG ^MCTOBKa: MH^opMaqwa 3a noTpe6MTena.........................12
UK, IE, MT The following information is intended
for healthcare professionals only.......................................15
HU Az alabbi informaciok kizarolag
egeszsegugyi szakembereknek szolnak...........................17
RO Urmatoarele informal sunt destinate
numai profesioniftilor din domeniul sanatajii.....................19
BG noco^eHaTa no-gony MH^opMaqwa
e npegHa3HaneHa caMo 3a Meg^quHCKM cneqwanwc™ 21
THIS PAGE IS INTENTIONALLY LEFT BLANK
Package leaflet: Information for the user
UK IE MT
BIPHOZYL
Solution for haemodialysis /
haemofiltration
Magnesium chloride hexahy-
drate, Sodium chloride, Sodium
hydrogen carbonate, Potassium
chloride, Disodium phosphate
dihydrate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
WHAT IS IN THIS LEAFLET
1. What BIPHOZYL is and what it is used for
2. What you need to know before you use BIPHOZYL
3. How to use BIPHOZYL
4. Possible side effects
5. How to store BIPHOZYL
6. Contents of the pack and other information
1. WHAT BIPHOZYL IS
AND WHAT IT IS USED FOR
This medicine is a solution for dialysis treatment (haemofiltration, haemodialysis and haemodiafiltra-tion) which is used to remove waste products from the blood when the kidneys are not functioning. This medicine is used in hospitals during intensive care treatment using Continuous Renal Replacement Therapy (CRRT). This medicine is particularly used to treat critically ill patients with acute kidney injury having:
• a normal concentration of potassium (normal kalaemia) in the blood
• a normal pH in the blood
• a normal or low concentration of phosphate (normal or hy-pophosphataemia) in the blood
• a high concentration of calcium (hypercalcaemia) in the blood
2. WHAT YOU NEED TO KNOW BEFORE YOU USE BIPHOZYL
DO NOT USE BIPHOZYL IN CASE OF:
• allergy to one of the active substances or any of the other ingredients (listed in section 6)
• a low concentration of calcium (hypocalcaemia) in the blood
• a high concentration of potassium (hyperkalaemia) in the blood
• a high concentration of phosphate (hyperphosphataemia) in the blood
WARNINGS AND PRECAUTIONS Warnings
Talk to your doctor, pharmacist or nurse before using BIPHOZYL.
Because BIPHOZYL contains potassium, high blood potassium level may occur shortly after starting the treatment. Your doctor will decrease the infusion rate and confirm that the potassium concentration has returned to desired level. If the condition does not resolve, the doctor must stop the administration immediately.
Because BIPHOZYL contains phosphate, high blood phosphate level may occur shortly after starting the treatment. Your doctor will decrease the infusion rate and confirm that the phosphate concentration has returned to desired level. If the condition does not resolve, the doctor must stop the administration immediately.
Your doctor will regularly monitor electrolyte and blood acid-base parameters in patients treated with BIPHOZYL. BIPHOZYL contains hydrogen phosphate, a weak acid that can influence your acid-base balance. If a reduction of the plasma bicarbonate concentration develops or worsens during therapy with BIPHOZYL, your doctor will decrease the infusion rate. If the condition does not resolve, the doctor must stop the administration immediately.
The instructions for use must be strictly followed.
The solutions in the two compartments must be mixed before use. Use only with a dialysis machine for CRRT.
Use only if the overwrap and solution bag are undamaged. All seals must be intact. Use of a contaminated solution may cause sepsis and shock.
Use only with an appropriate extracorporal renal replacement equipment.
Precautions
This medicine is calcium free and could cause hypocalcaemia. Infusion of calcium might be necessary.
BIPHOZYL may be warmed to 37°C to enhance patient comfort. However, only dry heat should be used. Solutions should not be heated in water or in a microwave oven. BIPHOZYL should be inspected visually for particulate matter and discoloration prior to administration.Do not administer unless the solution is clear and the seal is intact.
Your doctor will closely monitor your haemodynamic status, fluid balance, electrolyte and acid-base balance throughout the procedure, including all fluid inputs (intravenous infusion) and outputs (urine output), even those not directly related to CRRT.
This medicine has a hydrogen carbonate content at the lower end of the normal concentration range in the blood. This is appropriate when using citrate anticoagulation, as citrate is metabolized to hydrogen carbonate, or when normal pH values have been restored. Assessment of buffer needs, through repeated measurement of blood acid/base parameter and review of the overall therapy, is mandatory.
A solution with higher hydrogen carbonate content may be required. In case of abnormally high volume of fluid in the body (hypervolae-mia), the net ultrafiltration rate prescribed for the CRRT device can be increased and/or the rate of administration of solutions other than replacement fluid and/or dialysate can be reduced.
In case of abnormally low volume of fluid in the body (hypovolaemia), the net ultrafiltration rate prescribed for the CRRT device can be reduced and/or the rate of administration of solutions other than replacement fluid and/or dialysate can be increased.
Children
No specific adverse effect on children is expected when using this medicine.
Older people
No specific adverse effect on older people is expected when using this medicine.
Other medicines and BIPHOZYL
Tell your doctor, pharmacist or nurse if you are taking, have recently taken or might take any other medicines including medicines obtained without a prescription. This is because the concentration of other medicines may be reduced during dialysis treatment. Your doctor will decide if any changes in the dosage of your medicines should be made. In particular, tell your doctor if you are using either of the following:
• Additional sources of phosphate (e.g. nutritional fluids); as this may increase the risk of a high concentration of phosphate in the blood (hyperphosphatemia).
• Vitamin D and medicinal products containing calcium chloride or calcium gluconate; as they can increase the risk of a high concentration of calcium in the blood (hypercalcaemia).
• Sodium bicarbonate; as this may increase the risk of excess of bicarbonate in your blood (metabolic alkalosis).
Pregnancy, breast-feeding and fertility
Pregnancy and breast-feeding: There is no documented clinical data on the use of this medicine during pregnancy and lactation.
This medicine should only be administered to pregnant and lactat-ing women if clearly needed. Fertility:
No effects on fertility are anticipated, since sodium, potassium, magnesium, chloride, hydrogen phosphate and hydrogen carbonate are normal constituents of the body.
Driving and using machines
This medicine is not known to affect the ability to drive or use machines.
3. HOW TO USE BIPHOZYL
For intravenous use and use in haemodialysis. This medicine is to be used in hospitals and administered by medical professionals only. The volume used, and therefore the dose of this medicine, will depend on your condition. The dose volume will be determined by your doctor.
Always use this medicine exactly as your doctor, pharmacist or nurse has told you. Check with your doctor, pharmacist or nurse if you are not sure.
It is the responsibility of the physician to determine the compatibility of an additive medication with this medicine by checking for possible colour change and/or possible precipitation. Before adding a medication, verify if it is soluble and stable in this medicine.
POSOLOGY
The range of flow rates when used as replacement solution in haemofiltration and haemodiafiltration are: Adult and
adolescents: 500 - 3000 ml/h
Children: 15 - 35 ml/kg/h
The range of flow rates when used as dialysate in continuous haemodialysis and continuous haemodia-filtration are:
Adult and
adolescents: 500 - 2500 ml/h
Children: 15 - 30 ml/kg/h
INSTRUCTIONS FOR USE
This medicine will be given to you in a hospital. Your doctor will know how to use it.
For instructions for use see the end of this leaflet.
IF YOU USE MORE OF BIPHOZYL THAN YOU SHOULD
Contact your doctor or nurse immediately if you have taken more of this medicine than recommended in this package leaflet or than prescribed by your doctor and you feel uncomfortable.
The symptoms of overdose are tiredness, oedema or shortness of breath.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them. Your blood tests and clinical condition will be regularly monitored by a doctor or nurse in order to find possible side effects. Use of this solution could cause:
• Changes of levels of salts in the blood (electrolyte imbalances) such as: low calcium level (hy-pocalcaemia), high potassium level (hyperkalaemia) and high phosphate level (hyperphospha-taemia)
• Reduction of the plasma bicarbonate concentration (metabolic acidosis)
There are also some side effects which can be caused by dialysis treatments, such as:
• Abnormally high (hypervolae-mia) or low volume (hypovolae-mia) of fluid in the body
• Decreased blood pressure
• Nausea, vomiting
• Muscle cramps
REPORTING OF SIDE EFFECTS
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via:
United Kingdom:
Yellow Card Scheme www.mhra.gov.uk/yellowcard
Republic of Ireland:
HPRA Pharmacovigilance,
Earlsfort Terrace, IRL - Dublin 2;
Tel: +353 1 6764971;
Fax: +353 1 6762517;
Website: www.hpra.ie;
E-mail: medsafety@hpra.ie
Malta:
ADR Reporting
Website: www.medicinesauthority. gov.mt/adrportal
By reporting side effects you can help provide more information on the safety of this medicine.
WHAT BIPHOZYL CONTAINS
Before reconstitution
In the small compartment, A (250 ml):
5. HOW TO STORE BIPHOZYL
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and the packaging. The expiry date refers to the last day of that month.
This medicinal product does not require any special storage conditions.
Do not freeze.
Chemical and physical in-use stability of the reconstituted solution has been demonstrated for 24 hours at +22°C. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and should not be longer than 24 hours including the duration of the treatment.
The solution can be disposed of via wastewater without harming the environment.
Do not use this medicine if you notice damage to the product or visible particles in the solution. All seals must be intact.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
Magnesium chloride hexahydrate 3.05 g/l
In the large compartment, B (4750 ml): Sodium chloride 7.01 g/l
Sodium hydrogen
carbonate 2.12 g/l
Disodium phosphate dihydrate 0.187 g/l
After reconstitution
The reconstituted solution, A+B: Active substances
The other ingredients are:
• Dilute hydrochloric acid (for pH adjustment) E 507
• Water for injections
• Carbon dioxide (for pH adjustment) E 290
WHAT BIPHOZYL LOOKS LIKE AND CONTENTS OF THE PACK
This medicine is a solution for haemodialysis / haemofiltration and is packed in a two-compartment bag of a multilayer film containing polyolefins and elastomers.
The final solution is obtained after opening the peel seal and mixing the solutions in the small and large compartments. The solution is clear and colourless. Each bag contains 5000 ml solution and the bag is overwrapped with a transparent film. Each box contains two bags and one package leaflet.
MARKETING AUTHORISATION HOLDER
Gambro Lundia AB Magistratsvagen 16 226 43 Lund Sweden
MANUFACTURER
Gambro Dasco SpA Via Stelvio, 94 23035 Sondalo (SO)
Italy
This medicinal product is authorised in the Member States of the EEA under the following names: Austria, Belgium, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, United Kingdom: BIPHOZYL
Bulgaria: BIPHOZYL (5w£o3nn) This leaflet was last revised in 07/2016
mmol/l mEq/l
|
Sodium, Na+ |
140 |
140 |
|
Potassium, K+ |
4 |
4 |
|
Magnesium, Mg2+ |
0.75 |
1.5 |
|
Chloride, Cl- |
122 |
122 |
|
Hydrogen phosphate, HPO42- |
1 |
2 |
|
Hydrogen carbonate, HCO3- |
22 |
22 |
|
Theoretical osmolarity: |
290 mOsm/l | |
pH: 7.0 - 8.0
Betegtajekoztato: Informaciok a felhasznalo szamara
HU
Biphozyl
Hemodializalo vagy hemofiltracios oldat Magnezium-klorid-hexahidrat, Natrium-klorid, Natrium-hidrogen-karbonat, Kalium-klorid, Dinatrium-foszfat-dihidrat Mielott elkezdi alkalmazni ezt a gyogyszert, olvassa el figyelmesen az alabbi betegtajekoztatot, mert az On szamara fontos informaciokat tartalmaz.
• Tartsa meg a betegtajekoztatot, mert a benne szereplo informaciokra a kesobbiekben is szuksege lehet.
• Tovabbi kerdeseivel forduljon kezeloorvosahoz, gyogysze-reszehez vagy a gondozasat vegzo egeszsegugyi szakem-berhez.
• Ha Onnel barmilyen mellekha-tas jelentkezik, tajekoztassa kezeloorvosat, gyogyszereszet vagy a gondozasat vegzo egeszsegugyi szakembert. Ez a betegtajekoztatoban fel nem sorolt barmilyen lehetseges mellekhatasra is vonatkozik. Lasd 4. pont.
A BETEGTAJEKOZTATO TARTALMA:
1. Milyen tipusu gyogyszer a Biphozyl es milyen betegsegek eseten alkalmazhato?
2. Tudnivalok a Biphozyl alkalmazasa elott
3. Hogyan kell alkalmazni a Biphozyl-t?
4. Lehetseges mellekhatasok
5. Hogyan kell a Biphozyl-t tarolni?
6. A csomagolas tartalma es egyeb informaciok
1. MILYEN TiPUSU
GYOGYSZER A BIPHOZYL ES MILYEN BETEGSEGEK ESETEN ALKALMAZHATO?
Ez a gyogyszer diatiziskezeleshez (hemofiltracio, hemodiatizis es he-modiafiltracio) valo oldat, amely a mar szuksegtelen anyagok eltavoti-tasara szolgal a verbol, ha a vesek nem mukodnek. Ezt a gyogyszert korhazban alkalmazzak az intenzw ellatas soran, folyamatos vesepotlo kezeles celjabol. Ezt a gyogyszert kulonosen olyan eletveszelyes allapotu, akut vesekarosodasban szenvedo betegek kezeleseben alkalmazzak, akiknel a kovetkezok allnak fenn:
• a ver normalis kaliumszintje (normokalemia),
• a ver normalis pH-szintje,
• a ver normalis vagy alacsony foszfatszintje (normofoszfatemia vagy hipofoszfatemia)
• a ver magas kalciumszintje (hiperkalcemia).
2. TUDNIVALOK A BIPHOZYL ALKALMAZASA ELOTT
NE ALKALMAZZA A BIPHOZYL-T A KOVETKEZO ESETEKBEN:
• allergia a gyogyszer valamelyik hatoanyagaval vagy (a 6. pont-ban felsorolt) egyeb osszetevo-jevel szemben,
• a ver alacsony kalciumszintje (hipokalcemia),
• a ver magas kaliumszintje (hiperkalemai)
• a ver magas foszfatszintje (hiperfoszfatemia).
FIGYELMEZTETESEK ES OVINTEZKEDESEK
Figyelmeztetesek A Biphozyl alkalmazasa elott be-szeljen kezeloorvosaval, gyogysze-reszevel vagy a gondozasat vegzo egeszsegugyi szakemberrel.
Csak akkor alkalmazza az oldatot, ha az atlatszo, es nincsenek benne lathato reszecskek.
A hasznalati utasitast szigoruan be kell tartani.
A ket rekeszben talalhato oldatokat felhasznalas elott ossze kell keverni.
Csak diatiziskeszulekkel vegzett folyamatos vesepotlo kezeleshez alkalmazhato.
Csak akkor hasznalhato, ha a kulso csomagolas es az oldatot tartalma-zo zsak nincs megserulve. Minden lezarasnak epnek kell lennie. Szennyezodott oldat alkalmazasa szepszist es sokkot okozhat.
A csatlakozok nem megfelelo meg-valasztasa vagy hasznalata, illetve a folyadekaramlas egyeb akadalya a beteg hibas testsulycsokkenesi ertekehez vezethet, ami a keszulek riasztasat valthatja ki. A kezeles
folytatasa a kivalto problema elharf-tasa nelkul a beteg egeszsegkaro-sodasat vagy halalat okozhatja.
Ovintezkedesek:
Ez a gyogyszer nem tartalmaz kalciumot, es hipokalcemiat okoz-hat. Szuksegesse valhat kalcium infuzioja.
Ha az oldatot testhomersekletre (+37°C) kell melegtieni, ezt a muveletet gondosan ellenorizni kell. Az oldat alkalmazasa elott meg kell gyozodni arrol, hogy az oldat tiszta, es nem lathatok benne reszecskek. Ha az oldat nem felel meg ennek a feltetelnek, el kell dobni.
A kezeloorvosa a beavatkozas soran szorosan monitorozni fogja az On hemodinamikai allapotat, folyadekegyensulyat, illetve elektro-lit- es sav-bazis-egyensulyat.
Ennek a gyogyszernek a hidro-gen-karbonat tartalma a ver normal koncentraciotartomanya also hataranak felel meg. Ez megfelelo a citrattal vegzett veralvadasgatlas eseten, mivel a citrat hidrogen-kar-bonatta metabolizalodik, vagy amikor a ver normal pH-erteket mar helyrealtitottak. A pufferszuk-seglet ertekelese a ver pH-janak ismetelt meresevel, es a terapia atfogo ertekelese elengedhetetlen. Szuksegesse valhat magasabb hidrogen-karbonat tartalmu oldat alkalmazasa.
A szervezet rendellenesen nagy folyadektartalma (hipervolemia) eseten a folyamatos vesepotlo kezelesben alkalmazott keszulekre elofrt netto ultrafiltracios sebesseg novelheto, es/vagy a szubsztituci-os oldat es/vagy a dializalo oldat kivetelevel az oldatok beadasi sebessege csokkentheto.
A szervezet rendellenesen ala-csony folyadektartalma (hipovole-mia) eseten a folyamatos vesepotlo kezelesben alkalmazott keszulekre elofrt netto ultrafiltracios sebesseg csokkentheto, es/vagy a szubsz-titucios oldat es/vagy a dializalo oldat kivetelevel az oldatok beadasi sebessege novelheto.
Gyermekek
E gyogyszer gyermekeknel valo alkalmazasakor nem varhatok a gyermekekre nezve specifikus mellekhatasok.
Idosek
E gyogyszer idoseknel valo alkalmazasakor nem varhatok az idosekre nezve specifikus mellek-hatasok.
Egyeb gyogyszerek es a Biphozyl
Feltetlenul tajekoztassa kezelo-orvosat, gyogyszereszet vagy a gondozasat vegzo egeszseg-ugyi szakembert a jelenleg vagy nemregiben szedett, valamint szedni tervezett egyeb gyogysze-reirol; ez vonatkozik a veny nelkul kaphato gyogyszerekre is. Ez azert fontos, mert a dializiskezeles soran csokkenhet az egyeb gyogyszerek koncentracioja a szervezeteben. A kezeloorvosa el fogja donteni, hogy modosrtani kell-e gyogyszereinek adagjat.
Terhesseg, szoptatas es termekenyseg Terhesseg es szoptatas:
Nincsenek dokumentalt klinikai adatok a gyogyszer alkalmazasarol terhesseg vagy szoptatas alatt. Terhes vagy szoptato noknel csak akkor szabad ezt a gyogyszert alkalmazni, ha az egyertelmuen szukseges.
Termekenyseg:
Mivel a natrium, a kalium, a mag-nezium, a klorid, a hidrogen-foszfat es a hidrogen-karbonat a szervezet normalis alkotoreszei, nem varhato, hogy a gyogyszer hatassal lenne a termekenysegre.
A keszitmeny hatasai a gepjarmuvezeteshez es a gepek kezelesehez szukseges kepessegekre
Nem ismert, hogy ez a gyogyszer befolyasolna a gepjarmuveze-teshez es a gepek kezelesehez szukseges kepessegeket.
3. HOGYAN KELL ALKALMAZNI A BIPHOZYL-T?_
Intravenas alkalmazasra es hemodiaKzishez valo alkalmazas-ra. Ez a gyogyszer csak korhaz-ban alkalmazhato, es kizarolag egeszsegugyi szakember adhatja be. A beadott terfogat es ezzel a gyogyszer adagja az On allapotatol fugg. Az adagot a kezeloorvosa fogja meghatarozni.
A gyogyszert mindig a kezeloorvosa, gyogyszeresze vagy a gondozasat vegzo egeszsegugyi szakember altal elmondottaknak megfeleloen alkalmazza. Amennyi-ben nem biztos az adagolast illeto-en, kerdezze meg kezeloorvosat, gyogyszereszet vagy a gondozasat vegzo egeszsegugyi szakembert.
A kezelest vegzo orvos felelossege hogy megallapitsa a hozzaadott gyogyszernek a Biphozyl oldattal valo kompatibilitasat, az esetleges szinvaltozas es/vagy kicsapodas ellenorzesevel. Gyogyszer hozza-adasa elott gyozodjon meg arrol, hogy oldodik es stabil a Biphozyl oldatban.
ADAGOLAS
Hemofiltracio vagy hemodiafiltracio eseten szubsztitucios oldatkent alkalmazva az alabbi filtracios sebesseg alkalmazando:
Felnottek
es serdulok: 500 - 3000 ml/ora
Gyermekek: 15 - 35 ml/kg/ora
Folyamatos hemodiaKzis vagy folyamatos hemodiafiltracio eseten dializalo oldatkent (dializatumkent) alkalmazva az alabbi filtracios sebesseg alkalmazando:
Felnottek
es serdulok: 500 - 2500 ml/ora
Gyermekek: 15 - 30 ml/kg/ora
HASZNALATI UTAS^AS
Ezt a gyogyszert On korhazban fogja kapni. A kezeloorvosa tudni fogja, hogyan kell alkalmazni.
A hasznalati utasrtasokat lasd a betegtajekoztato vegen.
HA AZ EL6^RTNAL TOBB BIPHOZYL-T ALKALMAZOTT
Azonnal forduljon kezeloorvosahoz vagy a gondozasat vegzo egesz-segugyi szakemberhez, ha az eb-ben a betegtajekoztatoban ajanlott adagnal vagy az orvos altal rendelt adagnal tobbet kapott a gyogyszer-bol, es rosszul erzi magat.
A tuladagolas tunetei a faradtsag, az odema es a legszomj.
4. LEHETSEGES
MELLEKHATASOK_
Mint minden gyogyszer, igy ez a gyogyszer is okozhat mellekhata-sokat, amelyek azonban nem min-denkinel jelentkeznek. A lehetseges mellekhatasok eszlelese celjabol a vervizsgalatok eredmenyet es az On klinikai allapotat rendszeresen ellenorzi egy orvos vagy apolo. Az oldat alkalmazasa a kovetkezoket okozhatja:
• A verben levo sok koncentra-cioinak elteresei (az elektro-lit-egyensuly zavarai), ^gy az alacsony kalciumszint (hipokal-cemia), a magas kaliumszint (hiperkalemia) es a magas foszfatszint (hiperfoszfatemia).
Vannak olyan mellekhatasok, ame-lyeket a dializiskezeles okozhat, tobbek kozott a kovetkezok:
• a szervezet rendellenesen nagy folyadektartalma (hipervolemia) vagy rendellenesen alacsony folyadektartalma (hipovolemia),
• alacsony vernyomas,
• hanyinger, hanyas
• izomgorcsok.
MELLEKHATASOK
BEJELENTESE
Ha Onnel barmilyen mellekhatas jelentkezik, tajekoztassa kezeloor-vosat, gyogyszereszet vagy a gondozasat vegzo egeszsegugyi szakembert. Ez a betegtajekoztato-ban fel nem sorolt barmilyen lehet-seges mellekhatasra is vonatkozik. A mellekhatasokat kozvetlenul a hatosag reszere is bejelentheti az alabbi elerhetosegeken keresztul.
Orszagos Gyogyszereszeti es Elelmezes-egeszsegugyi Intezet Postafiok 450 H-1372 Budapest Honlap: www.ogyei.gov.hu A mellekhatasok bejelentesevel On is hozzajarulhat ahhoz, hogy minel tobb informacio alljon rendelke-zesre a gyogyszer biztonsagos alkalmazasaval kapcsolatban.
5. HOGYAN KELL A
BIPHOZYL-T TAROLNI?
A gyogyszer gyermekektol elzarva tartando!
A dmken es a csomagolason feltuntetett lejarati ido utan ne alkal-mazza ezt a gyogyszert. A lejarati ido az adott honap utolso napjara vonatkozik.
Ez a gyogyszer nem igenyel kulon-leges tarolast.
Nem fagyaszthato!
Az elkeszrtett oldat kemiai es fizikai stabilitasa 22°C-on 24 oran at igazolt.
Amennyiben nem hasznaljak fel azonnal, a felhasznalasig az eltar-tasi ido es a tarolasi korulmenyek a felhasznalo felelossege, es nem haladhatja meg a 24 orat, beleertve a kezeles idotartamat is.
Ez a gyogyszer csak egyszeri alkalmazasra szolgal. A fel nem hasznalt oldatot el kell dobni.
A hulladekka valt oldat a szenny-v^zbe engedheto, nem karos a kornyezetre.
Ne alkalmazza ezt a gyogyszert, ha a csomagolas serult, vagy ha az oldat lathato reszecskeket tar-talmaz. Minden lezarasnak epnek kell lennie.
6. A CSOMAGOLAS TARTALMA ES EGYEB INFORMACIOK
MIT TARTALMAZ A BIPHOZYL Elkeszites elott
A kis, „A” jelzesu rekeszben (250 ml):
magnezium-klorid-
A nagy, „B” jelzesu rekeszben (4750 ml):
natrium-hidrogen-karbonat 2,12 g/l kalium-klorid 0,314 g/l
dinatrium-foszfat-dihidrat 0,187 g/l
Elkeszftes utan Elkeszrtett oldat, az „A” es a „B” oldat kevereke: Hatoanyagok:
mmol/l mEq/l natrium (Na+) 140 140
hidrogen-
Elmeleti ozmolaritas: 290 mOsm/l pH = 7,0 - 8,0
Egyeb osszetevok:
• hrigrtott sosav (E 507)
(a pH beallrtasahoz)
• injekciohoz valo v^z
• szen-dioxid (E 290)
(a pH beallitasahoz)
MILYEN A BIPHOZYL KULLEME ES MIT TARTALMAZ A CSOMAGOLAS
Ez a gyogyszer hemodializalo / hemofiltracios oldat, amely poliolefi-neket es elasztomereket tartalmazo tobbretegu foliabol keszult ketre-keszes zsakba van csomagolva. A vegleges oldat elkeszitesehez fel kell nyitni a lezarast, es ossze kell keverni a kis es a nagy rekesz tar-talmat. Az ^gy keletkezo oldat tiszta es szmtelen. Egy zsak 5000 ml oldatot tartalmaz, es egy atlatszo vedocsomagolasban van. Egy doboz ket zsakot es egy betegtaje-koztatot tartalmaz.
A FORGALOMBA HOZATALI ENGEDELY JOGOSULTJA
Gambro Lundia AB Magistratsvagen 16 226 43 Lund Svedorszag
GYARTO
Telephely:
Gambro Dasco S.p.A.
Via Modenese 66 41036 Medolla (MO)
Olaszorszag
Gyartohely:
Gambro Dasco S.p.A.
Via Stelvio, 94 23035 Sondalo (SO)
Olaszorszag
OGYI-T-22818/02 2x5000 ml
ketrekeszes zsak injekcios csat-lakozoval (tuskecsatlakozoval) es szilikon gumibol keszult szeleppel ellatott Luer-csatlakozoval
Ezt a gyogyszert az Europai Gazdasagi Terseg tagallamaiban az alabbi neveken engedelyeztek: Biphozyl
A betegtajekoztato legutob-bi felulvizsgalatanak datuma:
2015. december
Prospect: Informatii pentru utilizator
RO
Biphozyl
Solutie pentru hemodializa/ hemofiltrare
Clorura de magneziu hexahidrat, Clorura de sodiu, Hidrogenocarbo-nat de sodiu, Clorura de potasiu, Fosfat disodic dihidrat Cititi cu atentie §i in Tntregime acest prospect Tnainte de a Tncepe sa utilizati acest medicament deoarece contine informatii importante pentru dumneavoastra.
• Pastraji acest prospect. S-ar putea sa fie necesar sa-l recitiji.
• Daca aveji orice Tntrebari supli-mentare, adresaji-va medicului dumneavoastra, farmacistului sau asistentei medicale.
• Daca manifestaji orice reacjii adverse, adresaji-va medicului dumneavoastra, farmacistului sau asistentei medicale. Aces-tea includ orice reacjii adverse nemenjionate Tn acest prospect. Vezi pct. 4.
CE GASIJI IN ACEST PROSPECT:
1. Ce este Biphozyl si pentru ce se utilizeaza
2. Ce trebuie sa stiji Tnainte sa utilizaji Biphozyl
3. Cum sa utilizaji Biphozyl
4. Reacjii adverse posibile
5. Cum se pastreaza Biphozyl
6. Conjinutul ambalajului si alte informajii
1. CE ESTE BIPHOZYL §I PENTRU CE SE UTILIZEAZA
Acest medicament este o solujie pentru tratamentul prin dializa (hemofiltrare, hemodializa si hemo-diafiltrare) care se utilizeaza pentru a elimina produsele reziduale din sange atunci cand rinichii nu funcjioneaza. Acest medicament se utilizeaza Tn spitale Tn trata-mentele de terapie intensiva prin terapie substitutiva renala continua (TSRC). Acest medicament se utili-zeaza Tn special pentru tratamentul pacienjilor bolnavi Tn stare critica cu insuficienja renala acuta care prezinta:
• o concentrajie normala de potasiu Tn sange (potasemie normala)
• un pH normal al sangelui
• o concentrajie normala sau mica de fosfat Tn sange (fosfa-temie normala sau hipofosfa-temie)
• o concentrajie mare de calciu Tn sange (hipercalcemie) 1
Precautii
Acest medicament nu conjine calciu si poate cauza hipocalcemie. Poate fi necesara administrarea de calciu.
Daca este necesara Tncalzirea solujiei la temperatura corpului (+37°C), procedura trebuie contro-lata cu atenjie. Tnaintea administra-rii trebuie verificat ca solujia sa fie limpede si sa nu prezinte particule. Tn caz contrar, eliminaji solujia la deseuri.
Medicul dumneavoastra va va monitoriza Tndeaproape starea hemodinamica, echilibrul fluidelor, echilibrul electrolitic si acido-bazic pe durata procedurii.
Acest medicament are un conjinut de bicarbonat minim cuprins Tn in -tervalul de concentrajii normale din sange. Acest aspect este favorabil atunci cand se utilizeaza anticoa-gulare cu citrat, deoarece citratul este metabolizat Tn bicarbonat, sau atunci cand valorile pH-ului au re-venit la normal. Evaluarea nevoilor de solujii tampon prin masurarea repetata a pH-ului sangelui §i evaluarea tratamentului Tn general este obligatorie. Poate fi necesara o solujie cu un conjinut mai mare de bicarbonat.
Tn cazul unui volum anormal de mare al lichidelor din corp (hiper-volemie), rata ultrafiltrarii nete pre-scrisa pentru dispozitivul de TSRC poate fi crescuta §i/sau viteza de administrare a altor solujii decat lichidul de substitujie si/sau dializat poate fi mic§orata.
Tn cazul unui volum anormal de mic al lichidelor din corp (hipovolemie), rata ultrafiltrarii nete prescrisa pentru dispozitivul de TSRC poate fi mic§orata §i/sau viteza de administrare a altor solujii decat lichidul de substitujie si/sau dializat poate fi crescuta.
Copii §i adolescent Nu se preconizeaza reacjii adverse specifice la copii si adolescenji cand se utilizeaza acest medicament.
Varstnici
Nu se preconizeaza reacjii adverse specifice la varstnici cand se utili-zeaza acest medicament.
Biphozyl impreuna cu alte medicamente
Informaji medicul dumneavoastra, farmacistul sau asistenta medicala daca luaji, aji luat recent sau aji putea lua orice alte medicamente, inclusiv medicamente eliberate fara prescripjie medicala. Concentra-jia altor medicamente se poate reduce Tn timpul tratamentului prin dializa. Medicul dumneavoastra va decide daca sunt necesare orice alte modificari Tn ceea ce privefte doza medicamentelor care vi se administreaza.
Sarcina, alaptarea §i fertilitatea
Sarcina fi alaptarea:
Nu exista date clinice documentate privind utilizarea acestui medicament Tn timpul sarcinii fi al alaptarii. Acest medicament trebuie adminis-trat femeilor gravide fi celor care alapteaza doar daca este absolut necesar.
Fertilitatea:
Nu se preconizeaza aparijia niciu-nui efect asupra fertilitajii, deoarece sodiul, potasiul, magneziul, clorul, hidrogenofosfatul fi hidrogeno-carbonatul (bicarbonatul) sunt constituent normali ai organismului uman.
Conducerea vehiculelor §i folosirea utilajelor
Nu exista date care sa indice ca acest medicament va afecteaza abilitatea de a conduce vehicule fi de a folosi utilaje. 2 te. Tnainte de a adauga un medicament, verificaji daca este solubil fi stabil Tn acest medicament.
DOZE
Viteza de perfuzare pentru solujia de substitute Tn hemofiltrare fi hemodiafiltrare este cuprinsa Tn urmatoarele intervale:
Adult
fi adolescenji: 500 - 3000 ml/ ora Copii: 15 - 35 ml/kg/ ora
Viteza de perfuzare pentru solujia de dializa (dializat) Tn hemodializa continua fi hemodiafiltrarea conti-nua este cuprinsa Tn urmatoarele intervale:
Adult
fi adolescenji: 500 - 2500 ml/ ora Copii: 15 - 30 ml/kg/ ora
INSTRUCJIUNI DE UTILIZARE
Acest medicament va va fi adminis-trat Tntr-un spital. Medicul dumnea-voastra va fti cum sa va adminis-treze acest medicament. Pentru instrucjiunile de utilizare consultaji partea finala a acestui prospect.
DACA UTILIZAJI MAI MULT BIPHOZYL DECAT TREBUIE
Contactaji imediat medicul sau asistenta medicala daca aji luat mai mult medicament decat doza recomandata Tn acest prospect sau decat v-a prescris medicul fi aveji o stare de disconfort.
Simptomele de supradozaj sunt oboseala, edemul fi dispneea.
4. REACJII ADVERSE
POSIBILE_
Ca toate medicamentele, acest medicament poate provoca reacjii adverse, cu toate ca nu apar la toa-te persoanele. Analizele dumnea-voastra de sange fi starea clinica vor fi controlate Tn mod regulat de un medic sau o asistenta medicala pentru a sesiza eventualele reacjii adverse. Utilizarea acestei solujii poate cauza reacjii:
• Modificari ale concentrajiilor sa-rurilor din sange (tulburari elec-trolitice) cum ar fi: concentrajie mica de calciu (hipocalcemie), concentrajie mare de potasiu (hiperpotasemie) fi concentrajie mare de fosfat (hiperfosfatemie)
De asemenea, pot sa apara unele reacjii adverse cauzate de trata-mentul prin dializa, cum ar fi:
• Volumul anormal de mare (hipervolemie) sau de mic (hipo-volemie) al lichidelor din corp
• Tensiune arteriala scazuta
• Greaja, varsaturi
• Crampe
RAPORTAREA REACJIILOR ADVERSE
Daca manifestaji orice reacjii adverse, adresaji-va medicului dumneavoastra, farmacistului sau asistentei medicale. Acestea includ orice reacjii adverse nemenjionate Tn acest prospect.
De asemenea, puteji raporta reacji-ile adverse direct prin intermediul Agenjia Najionala a Medicamentu-lui fi a Dispozitivelor Medicale Str. Aviator Sanatescu nr. 48, sector 1
Bucurefti 011478- RO Tel: + 4 0757 117 259 Fax: +4 0213 163 497 e-mail: adr@anm.ro Raportand reacjiile adverse, puteji contribui la furnizarea de informajii suplimentare privind siguranja acestui medicament.
5. CUM SE PASTREAZA
BIPHOZYL_
Nu lasaji acest medicament la vederea fi Tndemana copiilor.
Nu utilizaji acest medicament dupa data de expirare Tnscrisa pe eticheta fi pe ambalaj. Data de expirare se refera la ultima zi a lunii respective.
Acest medicament nu necesita condijii speciale de pastrare. A nu se congela.
Stabilitatea chimica fi fizica a solu-jiei reconstituite Tn timpul utilizarii a fost demonstrata pentru o perioada de 24 de ore la +22°C. Daca nu este utilizata imediat, perioada fi condijiile de pastrare Tnainte de utilizare devin responsabilita-tea utilizatorului fi nu trebuie sa depafeasca 24 de ore, incluzand Tn aceasta perioada fi durata tratamentului.
Acest medicament este destinat exclusiv unei singure administrari. Solujia neutilizata trebuie aruncata. Solujia poate fi eliminata Tn apa reziduala fara sa dauneze mediului Tnconjurator.
Nu utilizaji acest medicament daca observaji deteriorari ale medica-mentului sau particule vizibile Tn solujie. Toate sigiliile trebuie sa fie intacte.
CE CONJINE BIPHOZYL
inainte de reconstituire
Tn compartimentul mic, A (250 ml):
6. CONJINUTUL
AMBALAJULUI §i ALTE INFORMAJII_
Clorura de magneziu hexahidrat 3,05 g/l
Tn compartimentul mare, B (4750 ml): Clorura de sodiu 7,01 g/l
Hidrogenocarbonat de sodiu 2,12 g/l
Fosfat disodic dihidrat 0,187 g/l
Dupa reconstituire
solujia reconstituita, A+B: Substanje active
mmol/l mEq/l
|
Sodiu, Na+ |
140 |
140 |
|
Potasiu, K+ |
4 |
4 |
|
Magneziu, Mg2+ |
0,75 |
1,5 |
|
Clorura, Cl- |
122 |
122 |
|
Hidrogenofosfat, HPO42 Hidrogenocarbonat, |
1 |
2 |
|
HCO3- |
22 |
22 |
Osmolaritate teoretica: 290 mOsm/l pH = 7,0 - 8,0
Celelalte componente sunt:
• Acid clorhidric diluat
(pentru ajustarea pH-ului) E 507
• Apa pentru preparate injectabile
• Dioxid de carbon
(pentru ajustarea pH-ului) E 290
CUM ARATA BIPHOZYL §I CONJINUTUL AMBALAJULUI
Acest medicament este o solujie pentru hemodializa/hemofiltrare §i este ambalat Tntr-o punga cu doua compartimente, realizata dintr-o pelicula multistratificata care confine poliolefine §i elastomeri. Solujia finala este objinuta dupa desface-rea foliei de sigilare §i amestecarea solujiilor din compartimentele mic §i mare. Solujia este limpede §i incolora. Fiecare punga conjine 5000 ml de solujie, iar punga este Tmbracata Tntr-o pelicula transpa-renta. Fiecare cutie conjine doua pungi §i un prospect.
DEJINATORUL AUTORIZAJIEI DE PUNERE PE PIAjA §I FABRICANTUL
Dejinatorul autorizajiei de punere pe piaja Gambro Lundia AB Magistratsvagen 16 226 43 Lund Suedia
Fabricant
Adresa administrative:
Via Modenese, 66 Postcode 41036 Medolla (MO)
Italia
Loc de fabricatie:
Via Stelvio, 94
Postcode 23035 Sondalo (SO)
Italia
Acest medicament este autorizat Tn Statele Membre ale Spajiului Economic European sub urmatoarele denumiri comerciale: Biphozyl.
Acest prospect a fost revizuit Tn lunie 2015
MUBIAIBH 00 Bta 0>KOI/\I (fciW/\l0UOSOLI -MX) OiOUtTi a !OOHh01 OaiO0hMLTO>l oxomh OH0i/\idoHge eh MEhAuo g 'EiEEMUEMta MLTM/M IOOHh0I EiEHUOiMiOOIAIEE IO MHhMUEBd ‘01 -MdoaiEEd EH OHBJBUMdU EH EiiOOd -OXO MUBIAIBH 00 Bta MLTM/M ‘lyyQ EE BIBdBLIB BE BHBOMUtaodU ‘tTMh -BdiUMCfjBdiuA BH10H BH BiiOOdOXO MhMUOaA 00 Eb1 0>KOI/\I (t!MI/\l0LTOad0U -MX) OIOLTtTI a lOOHhOl OBiOOhMUOX oxoomb OH0i/\idoHgE eh MEhAuo g
IBHOgdBXH0JOdtaMX BH BMHEMLda.tfa.o oxoomb-ou o doaiEBd l/\IMtaOXgO0H 0 Bta 0>KO|/\| OMHOhOU OiOHiOOUtrh BH OMHOtaOlUgBH M El -a<idx eh yd eh OHBadoiAieM OHiBdx -OJOHIAI £0dh dOCf)Ag IO BiBtaxrfH MHohodu 00 Bta Bagtrdi ohlteimxl -LT<ltaBS Hd EH MiOOHMOiO 0IMHLTBI/\l -dOH BH 0HBat!aOHBIO£<ia Mdu MUM ‘iBHOgdBXHOJOdtaMX Ota Bta>KBdj£Bd 00 I<lIBdlMh OiBX M<li ‘0HBadMO<lO -OaMIOdU OHIBdiMh BH OHBaEUOUEM
Mdu otaitrtaoxtaou 0 01 Biaa.dx a
UMhEdiHOtlHOX BiBHUBIAldOH EH BhMHBdj ElEHUOtf a iEHOgdEXHOJ
-odtaVix EMLda.tfa.0 oaiodBxou Eaoi BdAtaohodu EiEutrh eh 0i/\i0da ou OHBUBg Mg UMHUEXUE-OHHMUOOMX M tTMHIMU -OdiXOUO ‘MlOOHhBl BH BOHBUBg ‘oAlBIO t!MHhMI/\IBHMtaOI/\l0X OHU0I -BIAIMH8 Mta0UO 0tal dBXOU itTMtalBg
BdoaiEBd 0i0ud<iax£M MEhAuo HOBMiOdU g MtlMiOBh Mi/\iMtaMa sag m daioMg 0 Bta Bagtrdi iadoaiEEd 0HEJEUMdU Mta0du OHUOiBIAIMHa EdMUOdiHOX 00 Eta
Bagtrdi BiBdAtaohodu ‘(o„/£+) Bd -AiEd0UI/\l0i EHO0U0i Ota BdoaiEBd EH 0HEatrdjE£ OI/\IMtaOXgO0H 0 OXV MMhUEX EH tTMEAcJjHM EI/\IMtaOXgO0H 0 Eta 0>KO|/\| tTMIAIOMh -UEXOUMX MHMhMdU Eta 0>KOI/\I M MMh
-ubx EMLda.tfa.0 eh oaiodBxou Eaoi
MXdSIAI MHEBUtaedU
BiHOMtlBU EH id<UAIO MUM trMHBtaxodaA ota otaoaota Bta o>koi/\i BiBHMhMdUOad<lU BH OHEdMJMdOX £0g OiOMHOhOU EH 0HBaE>KU<ltaodlJ MIAldEUE MHHMTT1BIAI EH OHBdMBMiXB MHMhMdU Bta OiOOX ‘BiHOMtlBU BH OUJ0i EH EgAiEE BHBUOXOH Ota iEtaOaOta Eta iBJOIAI ‘BiiOOHhOi BH Oi0MH0>KMata BH tTMHOhMHEdjO MiAdta mum ua.ioota be oiMdoaio EH BgodiOUA BiBHUMaBdUOH xom M OMOUOO MHMhMdU Bta 0>KOI/\I doaiEBd H0od<ll/\IB£ BH
Oi0HEa£UOU£l/| MHBiBhOUBE EO Eta Bagtrdi MxaoxBuo MXhMog MHotaoda -OU BO OH BdoaiEBd 0 EXEO M EiEX -aOXBUO OXB OIAIEO EaEUOUEM 00 Eft
■±yyO EE EEMUEMta EE iBdBUB 0 OIAIEO EaEUOUEM 00 Eft
tTMH0U
-otaio oioata a oiMdoaiEBd itrooiAio 90 Bta Bagtrdi BgodiouA Mtaody OHiXMdiO iEBEBUO 00 Eta Bagtrdi BgodiouA be 0iMMhxAdi0H|/|
MhMiOEh
Mi/\iMtaMa Eog m da.iOMg 0 iadoai -EEd OXE OIAIEO EaEUOUEM 00 Eft LTMEOCfjMg
0iEaeUOUEM Eta MtaodU ‘BdiO0O EXOHMhMtaOIAI MUM iaohBIAldBCj) ‘dBxou trMmBg o 0iMBai0a<iooij tTMHetaxraduAtaedu
MMd3l/\l MH£VUtf3dU M aMH3t/>K3dUAt/3dU
Bia<idx a
(tTMIAIOiBCfjOOCjjdOUMX) iBCfjOOCf)
BH tTMhBdiH0hHOX BX00M8 • !Bia<ldX a (trMIAI0MUBXd0UMX) MMLTBX BH tTMhEdiH0hHOX EX00M8 • !Eia<ldX a (tTMIAIOMhlJBXOUMX) MMhUEX EH tTMhEdiH0hHOX EXOMH • !(g EXhOi a MHOodgeM) MXaBiOa.0 OiMUBHBiOO iO 0 Bta M Oitrox IAI<IX MUM BaiO0tal0a OiMH -BMiXB iO 0OXtTH IAI<IX tTMidOLTB •
:VH MVhALfO
a LfM£Od>M9 aiMvacuducki 3H
m/i£o<Di/ig givaeuouen
Vtf l/ltf3dU ‘3±3VH£
_vP vaaKdi oaMVM z
Biaa.dx
a (tTMIAIOMhlJBXdOUMX) MMhUEX BH tTMhEdiH0hHOX EX00M8 •
lEia<idx
a (tTMIAIOiBCfjOOCjjOUMX MUM BHUBIAldOH) iBCfjOOCf) EH tTMhEdi -HOtlHOX EXOMH MUM BHUBIAldOH •
lBia<idx
a yd BH 08MH OHUBIAldOH •
lBia<idx
a (tTMIAIOMUBX BHUBIAldOH) MMUBX EH tTMhEdiHOhHOX BHUBIAldOH • iiBIAIM OiMOX ‘0iMh0dg<ig eh OHBtaxradaA odioo 0 MiHOMhEU MHUOg OHhMiMdX EH OMHOhOU EE OHiOdXHOX EaEUOUEM
90 oaiodExou Eaoi (±aao)
trMUBdOi BHU0iMiO0IAIB£ BHhOdg<ig
BHU0iMX<U<ltaodU BH BihlOIAlOU 0 OMHOhOU OHaMEHOiHM BH OlAIOda OU MhMHUog a BaeuoueM 00 oaiodsxou Eaoi iedMHOMhxHAcf) oh oiMhodg -<ig oiEiox ‘Eia<idx io MixAtaodu OiMHtaEUiO EH OHBatTHBdiOiO BE B8EUOUEM 00 OiMOX ‘(tIMhBdiUMCjjB -MtaOIAlOX M BEMUBMtaOIAlOX ‘tTMhEdi -UMCfjOIAlOX) OMHOhOU OHEMUBMta be doaiEBd 0 oaiodsxou Eaoi
vaeifouen go oa»v>i ve 1/1 UMEOdJug vaKifaviotfgdu oaMVM v
tTMhEIAldOCflHM EHU0iMHU<lUOta M BiBXaOXBUO EH 0MHEX<d<lta<lO 9 UMeocfiMg 0iBatrHBdx<io Bta xb>| g MMhXBOd MHBU0XOH MHXLOIAIE'ig p UMEOCflMg OiBaeUOUEM Bta XE>| e UMEOCflMg OiBaeUOUEM Eta Mtaodu ‘oiOEHe Eta Bagtrdi oaxs>| z EaEUOUEM 00 oaxsx BE M UMEoefjMg BatruaBiotaodu oaxB>| i
:V»a010MLJ MEVI V^dS-HS-O oaMVM
p BXhOi OiiKMg BXaOiOMU MEBi a MHEOMUOOH ‘MMhXBOd MHEU0XOH MHX<OIAie<ia MXhMoa m Eahoiuxa Eaoi ediooo EXOHMhMtaOIAI MUM iaotlBIAldBCf) ‘dBxou trMmBg oioiAiotaoaA ‘MMhXBOd MHOaiOdEXOU MHEU -0XOH MBXEXtTH OiMhAuOU OXV • Ediooo EXOHMhMtaOIAI MUM iaohBIAldBCf) ‘dBXOU trMmBg OiMBiMUOU ‘M00du<ia MHUOiMH -U<lUOta MaXBXtTH OiBIAIM OXV • oaoHio
OiOiOhOdU tr Bta MXLOUBH 00 Bta 0X<0|/\| BXaOiOMU MEBi OiOEEUEg • tSMhBIAldo4>HM OBg BE BHJKB3 BSKdS.taS.0 tSi OiBX MS.i ‘OSiOdBXSU BSOi SiBSCUOUCM Bta SiSHhOUBC Bta Mtaadu ‘BXSOiOMU BiButsh OHuaiBiNMHa aiaiahody
0)EjpAnip o)Endsond tuniposia ‘opuomo tun!SSE)Od ‘o)EuoqjEO uo6ojpAn tunjpos ‘opuoiqo tunjpos ‘0)EjpAnExon opuoiqo Lun;s3uBe|/\| uo!jB.ij|!jouiaL| /s|sA|B|pouj0Bq .ioj uognios lAzoqdjg
iEdtaMXMta iECflOOCf) aOMdiEHMta ‘taMdOUX aOMUEX ‘iEHOgdEXHOJOdta -MX aOMdiEH ‘taMdOUX aOMdiEH ‘iEdtaMXEOXOX taMdOUX a0ME0HJE|/\| tSMhBdiUM4>OIAI0X /BEMUBMtfOIAISX BE dOSiCBy UMC04>Mg
9H oxv Oo22+ Hdu eoEh pz eH SMHSxaja.taoclu a doaieed UMHoa -lOJMdU EH lOOHUMgElO BHhMEMCf) M BHhMIAIMX 0 BHBdMdlOHOIAIOt!'
EaaeEdiAiEe 00 0H stf OMHSHBdX‘10 EH UMaOUOA MHUB -MhOUO B8X0MEM 0H OaiOdBXOU BaOJ_ +ISOSIAI
UMHOhOOOU 10 HOta UMHtaOUOOU
eh adsaojio looHtaoj eh i<ixodo BiBxaoxBuo m eioxmio Axda.a HEE -auogio ‘looHtaoj eh sxodo taouo OaiOdBXOU Eaoi OlMBaSUOUEM 0H
Shota EE OHU<lI -ootaoH ‘oiouiai eh EaaHEdxa.o 00 stf
uneodjug
31VaKHVdXq.O Vtf MVM S
OaiOdBXOU Baoi BH BllOOH -OBUOE0g OHOOHIO UMhBiAidocfxHM 0h0aou BH 0HBaBhAuOU BE OOHMdU aoao 010tasta sta 0I0>koi/\i ‘MMhxsod MHEUOJKOH OlBashigO'lO 01B>|
6qBpqvww\A UMSogoA I-17E06826SE+ : ^3i ‘UMcfjoo COCI- ‘8 oN ..ssAdj huiaieP“ uA
BlBaiOdBXOU OU UMtlHOJE BHUOIMH
-u<iue|/| a ‘oHBashigo'io be eiaioiomo
ElEHUEHOMhSH E0dh OHlXOdMta MMhXBOd MHEUOJKOH SlMhigO<lO Eta ohia.o 010>ko|/\j MMhxsod mhbltexoh bxsoiomu mebi a mheomuooh mhx<oiai -E<ia MXhMoa sahoiuxa Baoj_ Ediooo BXOHMhMtaOIAI HUM dBXOU UMITlBg oioiAiotaoaA ‘MMhxsod MHoaiodsxou MHEUOJKOH MaXBXUH OlMhAuOU OXV
MkiriW3d
MHVLf3>K3H VH 3HVaVtTigoq.O
MIAIEBUO • !0HBhl<ldaOU ‘OHOtaBJ • lOHBJtiUBH
010Ha<ldX BH OHEaEJKMHOU • loiouai
a lOOHhOl BH OaiOOhMUOX (tiMIAI -0UO8OUMX) OXOMH HUM (UMIAIOU -oadouMx) oxooms OHOiAidoHgs • idOIAlMdUBH OIEX ‘ElEEMUEMta10 ItiH -MhMdU 00 Eta 1BJ0IAI 011/1 OX ‘MMhXBOd MHBUOX<SH MOXtiH M ISaAaiOOhl'lO
(umiaioibcJ)
-OOCfjdOUMX) IBCfjOOCf) BH 08MH oxoowa m (uMiAioMUBxdouMx) MMUBX BH Oai/IH 0X00M8 ‘(tiMIAIS -MhUBXOUMX) MMtlUBX EH 08MH OXOMH dOIAlMdUBH 01EX (OHELT -sgoMta H0imjodix0U0) Eia<idx a MU00 BH E1E8MH a BHUIAIOdU •
iMHMhMdu sta ox<oiai doaiEEd meoi eh sisgodiou/; Ediooo BXOHMhMtaOIAI MUM dEX0U io isastaoiugEH 90 ohi OHaotaod 0MHaOlO<lO Mg OlOHhMHMUX M tIMH -BataOUOEM Mg 01MHa<ldX ‘MMhXBOd
MHEU0XOH MHX<OIAIE<ia EH OHBatia -OHBIOA BS BaBhAuOU MJ MXOOa oh oh Mxoduaa ‘MMhxsod mhbuoxoh BXMaeMtaodu sta ox<oiai oaiodBxou Baoi ‘EaiodExou MXhMoa oixe>|
MMn>IV3d
l/IHVU3)K3H MH>KOIAI£g.a fr
xAtaea.a
EH JMlOOtaOH MUM X010 ‘EdOI/\lA E0 OHBdMEOtaodU BH 01MIAI01UIAIMQ ■0MH0X<OUOUEBd0H
oiBaioaAh oxb m dsxou itiMmsg
UBOMUtaodU 0 010XU0X10 MUM BX801
-omu EiEhiaoioEH a Eah<idou0du 90 oioxuoxio ‘oaiodBxou Baoi 10 0h0aou MUE8EU0UEM 010 OXE ‘OHaEgEEOH Ediooo BXOHMhMtaOIAI mum dsxou tiMmsg o 90 0iox<d<iao
Ljucoong vcoH
Vl¥l/\IMH0Xg03H IO 3h390U MLfwaeLfouen gio oxv
EXaOlOMU MEBI EH KEdX a oiiKMa EgodiouA be MMhxAdioHM ES EaEUOUEM OJ Bb'XBX 0EHE dEX0U immeg EhMHUog a OHBJBUMdu otj’a.g Mg ofri oaiodBxou Baoi V93dlOUA VC MMlTHAdlOHM M/B>|/|LU 08-si.
M/ILU 00S2 - OOS :Mm0H0iM
MH10EdE<ig :B0 KMhEdlUMCflEMtj'OIAlOX EHU01 -MX<U<lt3'odU M BEMUBMta'OIAlOX EHU01 -MX<U<lt3'odU Mdu (lEEMUEMf) 100H -h01 EHEMUEMf 01EX EgodlOUA MdU EX010U BH BlMgOtj' BH OlMHOEEUEMtj1
M/B>1/|LU se-si iehetr
M/ILU oooe - OOS :nmoHOi n
MH10Bd£<ig :E0 KMhEdlUMCflEMtj'OIAlOX M KMhEdlUMCflOIAlOX EE doaiEEd H0U01M1O0IAIEE oiEX EgodiouA Mdu EX010U BH BlMgOtj' BH OlMHOEEUEMtj1
vxaoducoH
oaiodBxou
Baoi a HOUMgEio m i/UMdoaieEd 0 M01 0h ‘010d0aA 00 ‘iHOIAlEXMtfOIAI oiMaEgotj1 eV Mtj’odu 'OHEaaEiA mum /M Eioah EH EHKIAIOdU EHX<OIAie<ia be Mdoaodu oibx ‘oaiodBxou Baoi 0 IHOI/llBXMta'OIAI H0U01MHU<lUOt3' BH EllOOIAIMlO0IAia<lO EH OHKUOtj'odUO
be looHdoaono mooh ladBXOLf Ediooo BXO
-HMhMtj'OIAI MUM laohBIAldBCf) ‘dBXOU KMmBg 01MB1MUOU ‘ohl0H a MHdAlMO 010 OH OXV Ediooo BXOHMhMta’OI/ll mum laohBiAidBcf) ‘dsxou laMmsg UBEBX 0 Mg 01XBX OHhOl oaiodBX -0U Baoi OlMBaeUOUEM MJBHMg
dsxou tiMmsg 10 Houotj'oduo otj’a.g ohi Eissotj' eh laiAiogo Mg 01 -OMHaoioao 10 MOMasE ohi ‘oaiodsx -0U Baoi EH ElBEOtf OHUOlEaOtj'OUO M OaiOOhMUOX 010HEa£U0U£l/| MlOMUEMhOUO MXOHMhMtfOI/ll 10 EJEUMdu 00 eV m MhMHUog a oiaieo sasuouEM 00 sta1 sagtidi oaiodsxou saoi EEMUEMtaoiAiox Mdu EgodiouA EE M EgodiouA EHEOHOaEdlHM ES
uneodjug
givacifoucM vtf xvx e
MHMmEIAI 0
oiMiogsd m oiEdMcfiom Eta Mg eh -ooHgooouo Axda.a oaiodsxou saoi EH Mixocfjo EE UMH0taigO<lO EIAIUH MHMmEIAI O Biogsd M 0HBdM4>Om oiouai oi
-oxmoaoh a isaioaoMdu OHUBi/ndoH IBHOgdEXHOJOdtaMX M 1E4)OO4)H0J -OdtaMX ‘taVldOUX ‘MMEOHJEIAI ‘MMUBX ‘MMdlEH OIEX M<11 ‘ElOlMUMldOCf)
Axda.a Mixons isaxEho 00 oh
:ioiMUMido0 OlAIMtaOXgOOH OHMEdX 0 OXE OIAIEO MH0X< Mhl0IAld<lX M MHH0IAI -odg Mdu EJEUMdu 00 sta sagadi oaiodsxou saoi OHOiAidax m iooh -HoiAiodg eh oiAioda ou oaiodsxou saoi EH OlOHEaSUOUEM EE MHHEta MHhMHMUX MHEdMIHOIAlAxOta SO OH :0H0IAld<lX M lOOHHOIAIOdg ISlMUMldecf) M 0H0IAldS.X ‘lOOHH01AI0dg
Eaiodsxou
OlMlAdta EH ElEXaodMEOta MHOIAIOdU 00 Eta sagadi Musta Mmod otai dsx -OUlUMmsg 'OMHOhOU OlOHEMUEMtf EH OlAIOda OU 0OUEIAIEH Eta 0X<OIAI
Eia<idx a saiodsxou oiMiAdta eh ei
-UMhsdlHOhHOX OlOtalEE ‘OlAIMtaOXgO
-oh 0 saoi siuohod sog mhoi-iAuou ‘saMXEi OHUoiMhoiuxa ‘saiodsxou MlAdta M8XEXUH OlOIAIOMdU Eta OHX<OIAI£<ia 0 MUM MUEIAIOMdU 010 OdOXOEH ‘OlEIAIOMdU OXE ‘EdlOOO sxoHMhMtaoiAi mum laohsiAidscf) ‘dsxou tiMmsg oiMsdMiAidocfjHi/i UMEOCjjMg M BaiOdBXSU MlAdtf
lOEdea.a
Exoohdsio a sdox Mdu MMhxsod mh -EU0XOH MHlOdXHOX IBaXEhO 00 OH
oaiodsxou saoi eh sgodiouA Mdy ioBdEs.a BxoohdBio a sdox
shota Mdu MMhxsod mh -EU0>K0H MHlOdXHOX IBaXEhO 00 OH
oaiodsxou saoi eh sgodiouA Mdu Bhst/
ElEEMUEMta MUM/M lOOHhOl EIEHUOIMIOSIAIEE 10 MHhMUEEd ‘01 -MdoaiEEd EH OH EJEUMdu EH BllOOd -oxo MhMuoaA so Eta mum/m ‘lyyo EE ElEdEUE EE EHEOMUtaodU ‘UMh -BdlUMCfjBdluA EH loodoxo E1EH10H
mmol/l mEq/l
|
HaTpuw, Na+ |
140 |
140 |
|
Kanuw, K+ |
4 |
4 |
|
Mame3ui/i, Mg2+ |
0,75 |
1,5 |
|
Xnopug, Cl- |
122 |
122 |
|
XugporeH$oc$aT, HPO42- |
1 |
2 |
|
XugporeHKap5oHaT, HCO3- |
22 |
22 |
TeopeTu^eH ocMonapuTeT: 290 mOsm/l pH = 7,0 - 8,0
ce u3non3Ba BegHara, BpeMeTO uycnoBuaTa 3a ctxpaHeHue npegu ynoTpe6a ce onpegenaT ot noTpe6uTena, ho BpeMeTo He Tpa6Ba ga HagBumaBa 24 4aca, BKnro^MTenHo npogtnwnTenHocrra Ha ne^eHueTo.
ToBa neKapcTBo e caMo 3a egHo-KpaTHa ynoTpe6a. Heu3non3BaHuaT pa3TBop Tpa6Ba ga ce u3XBtpnu. Pa3TBoptT Mowe ga ce u3XBtpnu 4pe3 oTnagHUTe Bogu 6e3 3aMtp-caBaHe Ha oKonHaTa cpega.
He u3non3BaMTe ToBa neKapcTBo, aKo 3a6enewuTe HapymaBaHe Ha ^nocrra Ha onaKoBKaTa Ha npogyKTa unu BuguMu nacTu^u b pa3TBopa. Bcuhku onaKoBKu Tpa6-Ba ga ca 3ane^aTaHu. 3
KAK M3mEWflA BM®O3Mn M KAKBO CbfltPWA OnAKOBKATA
ToBa neKapcTBo npegcTaBnaBa pa3TBop 3a xeMoguanu3a/xe-Moi^unTpa^ua, onaKoBaHo b caK c gBe oTgeneHua, komto e u3pa5oTeH ot MHoronnacToB $unM, ctgtp-wa^ nonuone^uHu u enacToMepu. KpaMHuaT pa3TBop ce nony^aBa cneg oTBapaHe u cMecBaHe Ha pa3TBopuTe b ManKoTo u ronaMoTo oTgeneHue. Pa3TBoptT e 6ucTtp u 6e3^eTeH. BceKu caK ctgtpwa 5000 ml pa3TBop u e o6but b npo-3pa^eH $unM. BcaKa KyTua ctgtp-wa gBa caKa u egHa nucToBKa.
nPMTE^ATEn HA PA3PEWEHMETO 3A ynOTPEBA
Gambro Lundia AB Magistratsvagen 16 226 43 Lund l±lBe^ua
nPOM3BOflMTEn
Gambro Dasco S.p.A.
Via Stelvio, 94 23035 Sondalo (SO)
MTanua
To3u neKapcTBeH npogyKT e pa3-pemeH 3a ynoTpe5a b gtpwaBuTe nneHKu Ha EMn nog cnegHuTe uMeHa: ABcTpua, Benrua, Btnra-pua, Kuntp, MemKa peny5nuKa, repMaHua, flaHua, EcToHua, Pbp^ua, McnaHua, OuHnaHgua, OpaH^ua, Peny5nuKa XtpBaTcKa, yHrapua, MpnaHgua, McnaHgua, MTanua, fiaTBua, fiuTBa, TIiok-ceM6ypr, ManTa, HugepnaHgua, HopBerua, nonma, nopTyranua, PyMtHua, lllBe^ua, CnoBeHua, CnoBaKua, 06eguHeHoTo Kpan-ctbo: Biphozyl
flaTa Ha nocnegHo npepa3rne»ga-He Ha nucToBKaTa 10/2014
flpymTe ctcTaBKU ca:
• Pa3pegeHa conHa KucenuHa (3a perynupaHe Ha pH) E 507
• Boga 3a uHwe^uu
• BtrnepogeH guoKcug
(3a perynupaHe Ha pH) E 290
X...............................................................................................................................................................................................................................................................................................................................X
POSOLOGY_
The volume and rate at which BIPHOZYL is administered depends on the blood concentration of phosphate and other electrolytes, acid-base balance, and overall clinical condition of the patient. Administration (dose, infusion rate and cumulative volume) of BIPHOZYL should be established by a physician.
The range of flow rates when used as replacement solution in haemofiltration and haemodiafiltration are: Adult and
adolescents: 500 - 3000 ml/h
Children: 15 - 35 ml/kg/h
The range of flow rates when used as dialysate in continuous haemodialysis and continuous haemodia-filtration are:
Adult and
adolescents: 500 - 2500 ml/h
Children: 15 - 30 ml/kg/h
Commonly used flow rates in adults are approximately 2000 ml/h which correspond to a daily replacement fluid volume of approximately 20 - 25 ml/kg/h.
Paediatric population Children < 16 years of age: Evidence from clinical studies and experience suggests that use in the paediatric population is not associated with differences in safety or effectiveness.
Older people
Adults > 65 years of age:
Evidence from clinical studies and experience suggests that use in the elderly population is not associated with differences in safety or effectiveness.
OVERDOSE
SYMPTOMS OF OVERDOSE
Overdose of BIPHOZYL can lead to severe clinical condition, such as congestive heart failure, electrolyte or acid-base disturbances.
TREATMENT OF OVERDOSE
Hypervolaemia / Hypovolaemia
If hypervolaemia or hypovolaemia occur, instruction for handling of hypervolaemia or hypovolaemia in Warnings (Section 2) must be strictly followed.
Metabolic acidosis If metabolic acidosis and/or hyperphosphatemia occur in the event of an overdose, stop administration promptly. There is no specific antidote for overdose. The risk can be minimized by close monitoring during treatment.
PREPARATION
AND/OR HANDLING_
The solution in the small compartment is added to the solution in the large compartment after breaking the peel seal immediately before use. The reconstituted solution shall be clear and colourless. Aseptic technique should be used throughout administration to the patient.
Aseptic technique should be used throughout administration to the patient.
Use only if the overwrap is undamaged, all seals are intact, peel seal is not broken, and the solution is clear. Press bag firmly to test for any leakage. If leakage is discovered, discard the solution immediately since sterility can no longer be assured.
The large compartment is fitted with an injection port for the possible addition of other necessary drugs after reconstitution of the solution.
It is the responsibility of the user to judge the compatibility of an additive medication with BIPHOZYL by checking for eventual colour change and/or eventual precipitation, insoluble complexes or crystals. Before adding a medication, verify if it is soluble and stable in this medicine and that the pH range of BIPHOZYL is appropriate (pH of reconstituted solution is 7.0-8.0). Additives may be incompatible. The instructions for use of the medication to be added must be consulted. Mix the solution thoroughly when additives have been introduced.
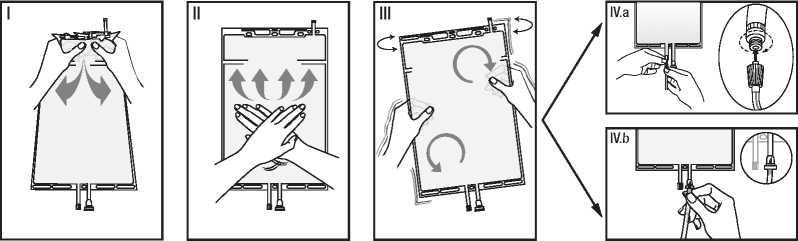
I Open the seal by holding the small compartment with both hands and squeezing it until an opening is created in the peel seal between the two compartments. (See figure I below.)
II Push with both hands on the large compartment until the peel seal between the two compartments is entirely open. (See figure II below.)
III Secure complete mixing of the solution by shaking the bag gently. The solution is now ready for use, and can be hung on the equipment.
(See figure III below.)
IV The dialysis or replacement line may be connected to either of the two access ports.
IV.a If the luer connector is used, remove the cap with a twist and pull motion, and connect the male luer lock on the dialysis or replacement line to the female luer connector on the bag using a push and twist motion. Ensure that the connection is fully seated and tighten. The connector is now open. Verify that the fluid is flowing freely. (See figure IV.a below.) When the dialysis or replacement line is disconnected from the luer connector, the connector will close and the flow of the solution will stop. The luer port is a needle-less and swabbable port.
IV.b If the injection connector (or spike connector) is used, first remove the snap-off cap. Introduce the spike through the rubber septum. Verify that the fluid is flowing freely.
(See figure IV.b below.)
The reconstituted solution is for single use only. Any unused solution must be discarded.
The solution can be disposed of via wastewater without harming the environment.
X...............................................................................................................................................................................................................................................................................................................................X
ADAGOLAS_
A Biphozyl ajanlott adagja a beteg klinikai allapotatol, az elektrolit- es a folyadekegyensuly celertekeitol, a pufferszukseglettol es az egyide-juleg szukseges egyeb oldatoktol fugg.
Ezert a dozist a kezelesert felelos orvosnak kell megallaprtania es elomia.
Hemofiltracio vagy hemodiafiltracio eseten szubsztitucios oldatkent alkalmazva az alabbi filtracios rata alkalmazando:
Felnottek
es serdulok: 500 - 3000 ml/ora
Gyermekek: 15 - 35 ml/kg/ora
Folyamatos hemodiaKzis vagy folyamatos hemodiafiltracio eseten dializalo oldatkent (dializatumkent) alkalmazva az alabbi filtracios rata alkalmazando:
Felnottek
es serdulok: 500 - 2500 ml/ora
Gyermekek: 15 - 30 ml/kg/ora
Felnottek eseteben az altalaban alkalmazott filtracios rata korul-belul 2000 ml/ora, amely napi 20 - 25 ml/kg/ora szubsztitucios oldatnak felel meg.
Gyermekek
16 evesnel fiatalabb gyermekek:
A klinikai vizsgalatok es tapasz-talatok eredmenyei alapjan a gyermekeknel torteno alkalmazas biztonsagossag es hatasossag vonatkozasaban nem kulonbozik a felnotteknel tapasztalhatotol.
Idosek
65 evesnel idosebb felnottek: A klinikai vizsgalatok es tapasztalatok eredmenyei alapjan az idoseknel torteno alkalmazas biztonsagossag es hatasossag vonatkozasaban nem kulonbozik a fiatalabb felnot-teknel tapasztalhatotol.
TULADAGOLAS
A TULADAGOLAS TUNETEI
A Biphozyl tuladagolasa sulyos allapotot, tobbek kozott pangasos szivelegtelenseget es az elektrolit- vagy a sav-bazis-haztartas zavarait okozhatja.
A TULADAGOLAS KEZELESE Hipervolemia
A tuladagolas eredmenyekent tudoodemaval jaro folyadektulter-heles es a pangasos szivelegtelen-seg mas tunetei jelentkezhetnek akut vagy kronikus veseelegtelen-segben szenvedo betegeknel. A hemodiaKzis, hemofiltracios vagy hemodiafiltracios kezeles folytata-saval novelheto az ultrafiltracioval eltavolrtott folyadek mennyisege; ezzel helyreallithato a normal folyadekegyensuly, es korrigalhato a tuladagolas.
Ezert hipervolemia eseten a folyamatos vesepotlo kezelesben alkalmazott keszulekre elofrt netto ultrafiltracios rata novelheto, es/ vagy a szubsztitucios oldat es / vagy a dializalo oldat kivetelevel az oldatok beadasi sebessege csokkentheto.
Hipovolemia
Hemofiltracio vagy hemodiafiltracio kozben fellepo sulyos hipovolemia eseten a folyamatos vesepotlo kezelesben alkalmazott keszulek-re elofrt netto ultrafiltracios rata csokkentheto, es/vagy a szubsztitucios oldat es/vagy a dializalo oldat kivetelevel az oldatok beadasi sebessege novelheto.
ELOKESZiTES ES/VAGY KEZELES_
Kozvetlenul a hasznalat elott tavo-lrtsa el a zsakrol a kulso csomago-last. Az oldat beadasa soran vegig aszeptikus technikat kell alkalmaz-ni. A mikrobiologiai kontaminacio elkerulese erdekeben az oldatot a felnyitasa utan azonnal fel kell hasznalni.
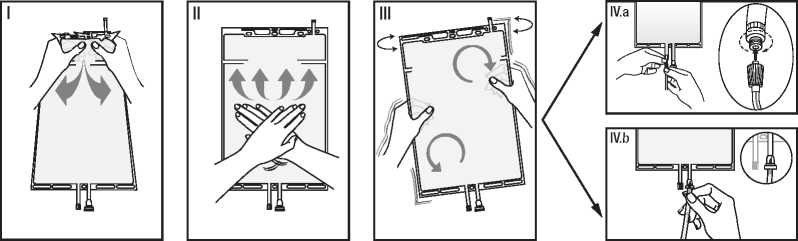
I A rekeszeket elvalaszto lezaras megnyitasahoz fogja meg a kis rekeszt ket kezzel, es nyomja ossze, amrig nyflas nem kelet-kezik a ket rekesz kozotti, fel-nyithato lezarason.
(Lasd az alabbi I. abrat.)
II Ket kezzel nyomja ossze a nagy rekeszt, amig a ket re-kesz kozotti, felnyithato lezaras teljes hosszusagaban meg nem nyflik.
(Lasd az alabbi II. abrat.)
III A zsak ovatos razasaval gon-doskodjon az oldatok maradek-talan osszekeveredeserol. Ez-zel az oldat keszen all az alkal-mazasra, es felfuggesztheto a megfelelo eszkozre.
(Lasd az alabbi III. abrat.)
IV A dializalo vagy szubsztitucios oldat vezeteke csatlakoztathato a ket kimeneti csatlakozo bar-melyikehez.
IV.a A Luer-csatlakozo hasznalata eseten, csavaro es huzo moz-dulattal tavolitsa el a sapkat, majd nyomo es csavaro moz-dulattal csatlakoztassa a diali-zalo vagy szubsztitucios oldat vezeteken talalhato dugos Luer-lock csatlakozot a zsakon talalhato Luer-csatlakozos al-jzatba. Ellenorizze, hogy a csatlakozok teljesen illeszked-nek-e egymashoz, majd szorit-sa meg. Ezzel a csatlakozo nyitva van. Ellenorizze, hogy a folyadek szabadon aramlik-e. (Lasd az alabbi IV.a abrat.)
Ha a dializalo vagy szubsztitucios oldat vezeteket lecsatla-koztatjak a Luer-csatlakozorol, a csatlakozo elzarodik, es az oldat aramlasa leall. A Luer-csatlakozo tu nelkuli, leto-rolheto csatlakozo.
IV.b Az injekcios csatlakozo (mas neven tuskecsatlakozo) hasz-nalata eseten, eloszor tavolrtsa el a lepattinthato sapkat. Szurja be a tusket a gumi valasztofa-lon keresztul. Ellenorizze, hogy a folyadek szabadon aramlik-e. (Lasd az alabbi IV.b abrat.)
X...............................................................................................................................................................................................................................................................................................................................X
DOZE_
Cantitatea de Biphozyl care va fi utilizata depinde de starea clinica a pacientului, de echiMbrul optim de electroliji fi fluide, de nevoile de solujii tampon fi de alte solujii care trebuie administrate concomitent. Prin urmare, stabilirea dozei rama-ne la discrejia medicului responsa-bil fi trebuie prescrisa de acesta.
Viteza de perfuzare pentru solujia de substitujie Tn hemofiltrare fi hemodiafiltrare este cuprinsa Tn urmatoarele intervale:
Adulji
fi adolescenji: 500 - 3000 ml/ ora Copii: 15 - 35 ml/kg/ ora
Viteza de perfuzare pentru solujia de dializa (dializat) Tn hemodializa continua fi hemodiafiltrarea conti-nua este cuprinsa Tn urmatoarele intervale:
Adulji
fi adolescenji: 500 - 2500 ml/ ora Copii: 15 - 30 ml/kg/ ora
Debitul utilizat frecvent la adulji este de aproximativ 2000 ml/ ora, valoare ce corespunde cu substitui-rea zilnica a unui volum de lichid de aproximativ 20 - 25 ml/kg/ ora.
Copii fi adolescenji Copii cu varsta sub 16 ani:
Datele objinute din studiile clinice fi experienja arata ca utilizarea la populajia pediatrica nu este asoci-ata cu diferenje Tn ceea ce privefte siguranja sau eficacitatea.
Varstnici
Adulji cu varsta peste 65 de ani: Datele objinute din studiile clinice §i experienja arata ca utilizarea la populajia varstnica nu este asocia-ta cu diferenje Tn ceea ce privefte siguranja sau eficacitatea.
SUPRADOZAJ
SIMPTOMELE DE SUPRADOZAJ
Supradozajul cu Biphozyl poate duce la o stare clinica severa, cum ar fi insuficienja cardiaca congesti-va, tulburarile echilibrului electrolitic sau acido-bazic.
TRATAMENTUL
SUPRADOZAJULUI
Hipervolemie
Supradozajul care se manifesta prin excesul de fluide cu edem pul-monar §i alte semne de insuficienja cardiaca congestiva poate aparea la pacienjii cu insuficienja renala acuta sau cronica. Continuarea tratamentului cu hemodializa, hemofiltrare sau hemodiafiltrare poate fi efectuata pentru crefterea volumului de lichid eliminat prin ultrafiltrare, pentru restabilirea echilibrului normal al fluidelor fi corectarea supradozajului.
Afadar, Tn cazul hipervolemiei, rata ultrafiltrarii nete prescrisa pentru dispozitivul de TSRC poate fi crescuta fi/sau viteza de adminis-trare a altor solujii decat lichidul de substitujie fi/sau dializat poate fi micforata.
Hipovolemie
Tn cazul hipovolemiei severe Tn tim-pul hemofiltrarii sau al hemodiafil-trarii, rata ultrafiltrarii nete prescrisa pentru dispozitivul de TSRC poate fi micforata fi/sau viteza de admi-nistrare a altor solujii decat lichidul de substitujie fi/sau dializat poate fi crescuta.
PREPARARE §I/SAU MANIPULARE_
Tndepartati ambalajul pungii imediat Tnainte de utilizare. Administrarea la pacient se va face folosind o tehni-ca aseptica. Solutia trebuie utilizata imediat dupa deschidere pentru a evita contaminarea microbiologica.
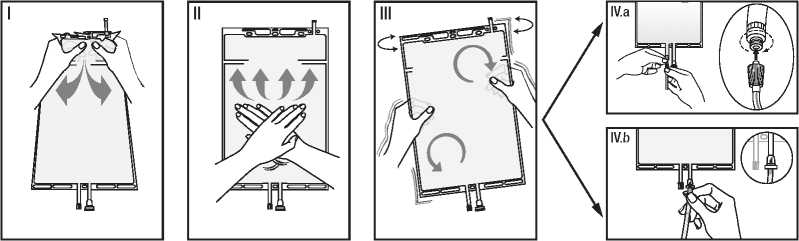
I Desfaceti folia protectoare ti-nand de compartimentul mic cu ambele maini §i presandu-l pana cand se creeaza o des-chizatura Tn folia de sigilare dintre cele doua compartimen-te. (Vezi figura I. de mai jos)
II Presati cu ambele maini compartimentul mare pana cand folia de sigilare dintre cele doua compartimente este complet deschisa.
(Vezi figura II. de mai jos)
III Asigurati amestecarea comple-ta a solutiei, scuturand u§or punga. Solutia este gata de uti-lizare §i poate fi agatata de echipament.
(Vezi figura III. de mai jos)
IV Linia de dializa sau de Tnlocuire poate fi conectata la oricare din cele doua porturi de acces.
IV.a Daca se utilizeaza conectorul luer, Tndepartati capacul cu o mifcare de rasucire §i tragere §i conectati conectorul luer lock tata (cu filet exterior) de pe linia de dializa sau de Tnlocuire la conectorul luer mama (cu filet interior) de pe punga utilizand o mifcare de Tmpingere §i ra-sucire. Asigurati-va de corecti-tudinea §i strangerea perfecta a conexiunii. Conectorul este acum deschis. Asigurati-va ca fluidul curge liber.
(Vezi figura IV.a de mai jos)
Daca linia de dializa sau de Tn-locuire este deconectata de la conectorul luer, conectorul se va Tnchide §i fluxul de solutie se va opri. Portul luer este un port fara ac §i poate fi curatat cu un betisor cu vata.
IV.b Daca se utilizeaza conectorul pentru injectie (sau conectorul cu varf ascutit), Tndepartati mai Tntai capacul cu deta§are automata. Introduce^ conectorul cu varf ascutit prin peretele des-partitor din cauciuc. Asigu-rati-va ca fluidul curge liber. (Vezi figura IV.b de mai jos)
eieemeM mjm/m iooHhsi eiEHUsimosiAiee io MHhmjEed ‘oiwdoaieBd eh eh -EJELTMdU EH BHOOdOXO m-iMLTOaA 90 bV HLTM/M ‘lyyo be eiedeLie be EHEOMUtfodU ‘KMhBdlLTMCflBdlLrA BH10H BH BHOOdOXO MLTBIAIBH 00 Ef 0>KOI/\I KMhBdlLTMCflBMtj'OIAlOX HUM KMhBdlLTMCflOIAlOX BH OlAIOda OU aWAIOLTOaOUMX BXXOI BH MBhAlJO g HHlM0LfOaOUHX
EIEEHLTEHtj'
MLTM/M lOOHhOl EIEHLT0imO0l/\IEE 10 MHhMLIEEd ‘OIMdoaiEBd EH 0HEJELT -MdU EH BHOOdOXO MLTBIAIBH 00 BV MLTM/M lyyO EE BIBdBUB BE BHBO -Mutfodu ‘aMhBdiuMcfiBdiLrA bhioh BH BHOOdOXO MhMLTOaA 00 BV 0>KOI/\I BMiAiouoadouMX bh mbtiAlto a bxbj_ 'OIOHEdMEOtfodU BdMJMdOX BV OIOOX ‘mOOHhOI BH OHBLTBg BMHLTBIAldOH MaOHBIOE<ia 00 BV M BMhBdlLTMCfjBdlLrA E0dh IOOH -hOl BHBdMHMIAIMLTO OIOaiOOhMLTOX MhMLTOaA 00 BV BS ‘MXLLT'lta'odU BV 0>KOI/\I BMhEdlLTMCfjEMta'OIAlOX HUM BMhBdlLTMCfjOIAlOX ‘EEMLTEMta'OIAlOX 0 OIOMHOhOLf 'lOOHha.lBlOOtfOH BHhOtj’da.O BHMOIOBE BH MtlBHEMdU
MjAdtj1 m xoio Hogodtatouog io oho>k -AdtaVidu ‘looHhoi o OHEadEaoiodu MHMhMdU BV 0>KOI/\I lOOHh'LIBIOOb'OH EHh0dg<ig BHhMHOdX HUM EdiOO 0 MIHOMtlBU MdU OIOHEdMEOtfodU HHlM0LfOad0UHX
3HVdM£Otf3dU MdU 3MH3h3U
OHELTEg BMHaOHOO-OHHMLTOOMX mum aMHiMiiodixouo a BMHomAdBH M lOOHha.IEIOOt'OH BHhOb'd‘10 EH -MOIOBE dOIAlMdUBH OIBX OMHBOIO<lO OHhMHMLTX OXXOI Ot?1 OtfOaOtf BV 0>KOI/\I LTMEOCfjMg BH OIOHEdMEOtfodU 3HVdM£Otf3dU VH MIAIOIUIAIMO
3HVdM£Otf3dU
BIIOOHOBXMCfjO HUM BIIOOHOBUOEOg a BMhMLIEBCl 0 BHBEd<iaO 0 0H J.OBdE'ia EXOOhdEIO a sdox io BMhBLrAuou Mdu BiBgodi -OUA Oh ‘lEJELTOUtfodU IMUO M BMHB8 -hAodU OIMHhMHMLTX IO OIMIBIIlAeod iMHHtatoj gg Ij'eh MHioBde<ig i0EdE<ia ExoohdEio a Edox
BIIOOHOBXMCfjO HUM BIIOOHOBUOEOg a BMhMLIEBd o EHEEd<iao 0 oh BMhBLrAuou eh -hMdlEMtfOU Hdu EIEgodlOUA 0h ‘iBJBLTOUb'odU IMUO m BMHBahAodu OIMHhMHMLTX IO OIMIBIIlAeod iMHHtatoj 91. tabu i0EdE<ia eh shop BMhBLfAuOU BHhMdlBMb'OU
M/B>|/|LU qz - oz
OHLTOIMEMLTgMdU IO lOOHhOl EH -LTOIMIOOIAIBE OaiOOhMLTOX OHaOHtj'
bh Baioioaioao oioox ‘n/|w 0002 OHLTOiMemjgMdu 0 MHioBde<ia Mdu BXOIOU BH ingot1 HBaELTOUEM OIO0h
M/6>1/|UJ oe-Sl^
M/ILU oosz - 00s :um0Ha m
MHioEdeag :eo BMhBdiiJMcJjBMta'oiAiox ehltoi -MXLLT'lta'odU M BeMLTBMb'OIAlOX EHLTOI -MXOT'lta'odU Hdu (lEEMLTEMta1) IOOH -hOI EHEMLTEMta1 OIBX EgodlOUA Mdu BXOIOU EH BIMgOb' BH OIMHOEEUEMt/1
M/B>1/|LU se-si iehetr
M/ILU oooe - 00S :um0Ha m
MHioBdeag :BO BMhBdlLTMCfjBMta'OIAlOX M BMhBdlLTMCfjOIAlOX be doaiEEd HOLTOimooiAiEE oibx EgodiouA Hdu BXOIOU EH BIHgOf EH OIMHOEEUEMt/1
dBXOLT awfriBa -AXOLT BH OMHEOMUtfodU M BXHOhodU
ou auotj'oduo 00 eibeo^ bboibs
OlAIMb'OXgOOH 0 BV 0X<OI/\I OMHOXLOLTMdU OHHOIAIOdaOHtj'O
0101/ih ‘wdoaiEEd mAdtj'm docfiAg 10 eieIj^kAh ‘mooHhoi m mmiodixoLTO EH OHELTEg aMBOLTOh ‘BIHOMhBU BH 0MHaOIO<lO OIOHhMHMLTX IO MowaBE ‘EasLrouEm oo bV Eagadi OI0OX ‘LTMEOCflMg OIOaiO0hMLTO>|
V»aOdM£OH
oa
momjBMhauo MMOHuhutfaw B£ owbo BHahBHBBHb'adu a
BMhBWdoebHM ALfOtf-OU BlBHahOOOM
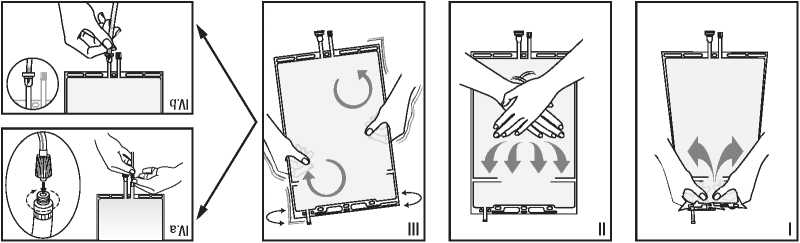
(Auob-ou q Al BdAjMcf) wg) OHbogoao umiab 00 eiiooHhsi 9h ‘00 0i0d0a/; EMuemAuee EIEH0I/\|Aj £0du BIBUJM BIMBdBM -odu BXhBUBM 00 BIBfrlBauAhiO 0i0HEdioio oada.u ‘(doi>i0HO>i aMH0dlOOBE MUM) 0HEdMl»0>KHM EE EdOl»0HO» 0IE8EUOUEM OMVq'AI HOU
-iaiei o EaiogEdgo 00 Bb 0>koi/\i m eujm E0g 0 doaio laMaodaAu ■©duo ©hi EdoaiEEd eh i<i>ioi -ou m MdoaiEE 00 0hl MOl ‘doi -»0HO» aMBOdoAu 10 BH0U0bEBd 0b<ig aMHMU EIEHU0IMIO0IAI -EE MUM ElEHEMUEMb OIBJO>|
(Auob-ou e Al EdAjMcf) >Kg) OHbogoao M>KMab 00 BIIOOHh0I ©h ‘00 0i0d0aA H0doaio 0 i<id -O1X0HOX EJ0Q EIEHJ0IO M EH -0HI<lUUA OHU<lUEH 0 BIEMS'lda 0h ‘00 0I0d08A '01Mld<iaEE M 010HOM1EH OJ 01EX ‘EX BO EH doi -X0HOX aodoAu aMXOH0>K 0 KMH -MU EIEHU0IMIO0IAIEE MUM E1EH -EMUEMb EH XMHMBdXBH aodoAu frlBahOtUXEE aMX>K‘ll/\l 0I0>Kd<iaO ■0IEUd‘lbEM M 0IMid<iaBE a OIBX ElEXhEUEX 010UB8O ‘dOIX0H -OX KMaodoAu 0IB8EUOUEM OXVBAI
ua.ioob be edoaio eieaf io 0 Bb m oimox i/\ia.x BHBeda.ao 0ba.g eta1 0>koi/\i kmhmu bibh
-U0IMIO0IAIBE MUM BIBHEMUBMtj1 Al
(Auob-ou hi BdAjMcf) x<g) oiBHBabAdogo Axda.a HOhBxo 0ba.g Bb 0>koi/\i m Bg0diouA be aoioj 0 0110a iadoaiEBd BXBO O»0U 0IMI -BUXEBd OIBX H0O0IAIO OHU<lUBH 0 ladoaiEBd ©h ‘00 0i0d0aA III
(Auob-ou || BdAjMcf) x<g) OHU<lUBH Mdoaio 00 aMHOuobio 0108b Abx<8i/\i oiomh -0hi<iuuA a ladoaio oiBxob ‘01 -0HOMiMdu oj m oh<id oaf 0 omh -auabio oioiAiauoj 010HOMIBH || (Auob-ou | BdAjMcf) x<g) aMHauabio aiaab Abx<ai/\i oiaMHaHia.uuA a doaio BaAsedgo 00 oibx -of ‘oioHOMiMdu oj m oh<id oab 0 0MH0U0biO OIOXUBIAI 0I0HB8X
oibx oi0MH0Hi<iuuA oiodoaio I ■0HBaaod<ll/\IBE OHhMJ -OUOMgodXMlAI OHJOgSM 00 Bb BE ‘Al/\l oiEHadBaio bauo BJBHbaa bbeuou -EM 00 Bagadi i<idoai£Bd bxmhxbi BHhMiuooB BaeuoueM 0O sb Bagadi BIHBMhBU BH 0MH0>KOUMdU Md|J BgodiouA Mbodu OHoaiobodoouoH ex bo io BiBxaMago aiBHBdioio
vioavd vh
HI/lhVH l/llfl/l/l/l VNaOlOJtfOU
THIS PAGE IS INTENTIONALLY LEFT BLANK

Gambro and Biphozyl are trademarks of Baxter International Inc., or its subsidiaries


CD
<
2 o
_Q CD
I O
O CL
CE TREBUIE SA §TIJI INAINTE SA UTILIZAJI BIPHOZYL_
NU UTILIZATI BIPHOZYL IN CAZ DE:
• alergie la una dintre substanjele active sau la oricare dintre ce-lelalte componente (enumerate la pct. 6)
• o concentrajie mica de calciu Tn sange (hipocalcemie)
• o concentrajie mare de potasiu Tn sange (hiperpotasemie)
• o concentrajie mare de fosfat Tn sange (hiperfosfatemie)
ATENJIONARI §I PRECAUJII
Atentionari
Tnainte sa utilizaji Biphozyl, adresaji-va medicului dumneavoas-tra, farmacistului sau asistentei medicale.
Utilizaji doar daca solujia este limpede si nu prezinta particule vizibile.
Instrucjiunile de utilizare trebuie respectate cu stricteje.
Solujiile din cele doua comparti-mente trebuie amestecate Tnainte de utilizare.
A se utiliza numai Tmpreuna cu un aparat de dializa pentru TSRC. Utilizaji doar daca ambalajul si punga de solujie nu prezinta deteriorari. Toate sigiliile trebuie sa fie intacte. Utilizarea unei solujii contaminate poate cauza septice-mie si soc.
Utilizarea incorecta a porturilor de acces sau a altor restricjii pentru fluxul de fluid poate conduce la scaderea incorecta Tn greutate a pacientului si poate declansa alarme ale aparatului. Continuarea tratamentului fara sa se solujioneze cauza inijiala poate conduce la vatamarea sau decesul pacientului.
CUM SA UTILIZAJI
BIPHOZYL_
Destinat utilizarii intravenoase fi utilizarii pentru hemodializa. Acest medicament va fi utilizat numai Tn unitaji spitalicefti fi va fi adminis-trat exclusiv de catre specialifti din domeniul medical. Volumul utilizat fi, prin urmare, doza acestui medicament vor depinde de starea dumneavoastra. Volumul dozei va fi stabilit de medicul dumneavoastra.
Utilizaji Tntotdeauna acest medicament exact afa cum v-a spus medicul dumneavoastra, farmacistul sau asistenta medicala. Discutaji cu medicul dumneavoastra, farmacis-tul sau cu asistenta medicala daca nu sunteji sigur.
Este responsabilitatea medicului sa determine compatibilitatea unui medicament adaugat suplimentar cu acest medicament, verificand o eventuala modificare a culorii fi/sau prezenja unor eventuale precipita-
CtfltPWAHME HA OnAKOBKATA M flOnt^HMTE^HA MH$OPMA^Ma_
KAKBO CtfltPWA BM0O3Mn
npegu pa3TBapaHe B ManKoTo oTgeneHue A (250 ml): MarHe3ueB xnopug xeKcaxugpaT 3,05 g/l
B ronaMoTo oTflenem/ie B (4750 ml): HaTpueB xnopug 7,01 g/l
HaTpueB
xugporeHKap5oHaT 2,12 g/l
KanueB xnopug 0,314 g/l
fluHaTpueB $oc$aT guxugpaT 0,187 g/l
Cneg pa3TBapaHe npuroTBeHuaT pa3TBop A+B: Aktubhu Be^ecTBa