Boots Hair Loss Treatment Regular Strength
139.7 mm
300 mm


Information for the user
Hair Loss Treatment Regular Strength
(Minoxidil)
Read all of this leaflet carefully before you take this medicine because it contains Important Information you need to know.
This medicine is available without prescription, however, you still need to use this product carefully to get the best results from it. Keep this leaflet as you may need to read it again. Ask your pharmacist if you need more information or advice.
1. What is this medicine and what is it used for?
This medicine contains:
• minoxidil which acts on the scalp to help hair growth on the bald area,
This solution is used for the treatment of common hereditary baldness (a condition called alopecia androgenetica).
2. Is this medicine suitable for you?
Do not take this medicine if you:
• are allergic to minoxidil or any of the other ingredients
• suffer from high blood pressure, whether treated or not
• have scalp conditions, including psoriasis (an itchy inflammatory skin condition) or sunburn
• have a shaved scalp
• are using other medicines or occlusive dressings, such as plasters or air tight dressings, on your scalp
• are pregnant or breast feeding
• are younger than 18 years or older than 65 years.
If you are using other medicines that are applied to the scalp which contain any of the following ingredients, please see your doctor or pharmacist before taking this medicine:
• corticosteroids
• tretinoin
• dithranol
• petrolatum
• guanethidine.
These medicines may cause minoxidil to be absorbed quickly and increase the risk of side effects.
If you are taking any of the following medicines please see your doctor:
• medicines for high blood pressure that dilate (widen) the blood vessels, such as hydralazine.
Information about some of the ingredients in this medicine:
• Contains propylene glycol.
May cause skin irritation.
• Contains ethanol (alcohol).
Can cause burning and irritation to the eyes, if the product comes into contact with the eyes, broken skin or lining of the mouth, throat or nose, bathe the area with large amounts of cool tap water.
3. How to use this medicine
Before you start to use this medicine, check with your doctor that your scalp is normal and healthy. This medicine is for use on the scalp only. Do not apply it to other areas of the body. Make sure that the scalp is clean and dry before applying.
Men and women aged 18 to 65 years:
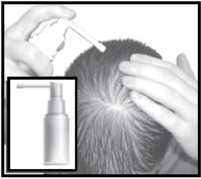
Apply 1 ml of the solution to the bald area twice daily, using the applicators provided (below).
Do not use more than 2 ml of the solution In 24 hours.
Pump spray applicator
Extended spray tip applicator

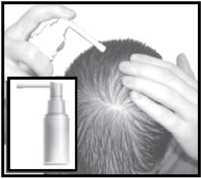
Dropper
This is useful for large areas of baldness.
•spray several times away from your head and body prior to first application to remove any airlocks •aim the pump at the centre of the bald area
•press once and spread the solution with your fingertips over the whole area
•repeat for a total of 6 sprays to apply the 1 ml dose •avoid breathing the spray mist.
Do not exceed the stated dose.
If you use too much of the solution by mistake, or if it is accidentally swallowed:
Contact your doctor or pharmacist straight away.
If you use your fingertips to rub the solution into your scalp, make sure that you wash your hands afterwards.
This is useful for small areas, or under the hair,
•the pump spray applicator must be in place to use this applicator •attach the extended applicator to the spray applicator •use in the same way as the pump spray applicator.
This is useful for small areas, •fill the dropper to the 1 ml mark
•apply the solution to the bald area until the dropper is empty.
i If the product comes into contact with the eyes, broken skin or lining of i ! the mouth, throat or nose:
1 Bathe the area with large amounts of cool tap water. 1
i If no re-growth occurs after 12 months use, stop using this medicine. ■
! The hair may take up to 4 months to re-grow, and in some people may not grow at all. The new hair ! ; may be fine hair, similar to baby hair. This medicine is a treatment and needs to be continued for hair ] i growth to be maintained. !
| If you stop the treatment, the new hair will disappear after 3 to 4 months j
i 4. Possible side effects
i Most people do not have any side effects while taking this medicine. However, if you experience any i | of the following side effects, or anything else unusual happens, stop using the medicine immediately, [ i and see your doctor or pharmacist.
If any of the below effects occur, seek medical advice immediately:
[• swelling of the face, lips, tongue and/or throat j
;• palpitations and/or fast heart beat
'• changes such as skin rash and redness leading to itching, dry and flaking skin and/or swelling where1 | the product is applied, or around the ears and face, blistering, bleeding and ulcers chest pain i
[• swelling in the feet and/or legs
i* sudden, unexplained weight gain i
'• dizziness, feeling faint due to low blood pressure. 1
J If any of the below effects occur, discontinue use and seek advice from ; ; your pharmacist:
! Very common (may affect more than 1 in 10 people):
Common (may affect up to 1 in 10 people):
!• unwanted hair growth on the face or body \
'• itching [• rash
Uncommon (may affect up to 1 in 100 people):
■• feeling sick and/or being sick ■
[• flaking skin, skin rash, dry skin or other skin problems such as dermatitis
i» acne (spots and oily skin) i
'• changes in hair colour/texture of the newty grown hair !• temporary hair loss.
I Reporting of side effects
| If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side j i effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme i ' at: www.mhra.aov.uk/vellowcard. 1
| By reporting side effects you can help provide more information on the safety of this medicine.
i 5. How to store your medicine
; Keep out of the sight and reach of children.
i Do not use this medicine after the expiry date printed on the pack.
1 Store upright, below 25°C and protect from light. 1
| When the bottle is empty, the bottle and applicators must be disposed of safety. j
! 6. What is in this medicine?
i Each 1 ml dose of clear solution contains the active ingredient: minoxidil 20 mg i
! The other ingredients are: propylene glycol, ethanol, water.
| This pack contains 60 ml of solution and 3 applicators. j
i 7. Who makes this medicine?
i The Marketing Authorisation holder and manufacturer is Wrafton Laboratories Limited, Braunton, i | Devon, EX33 2DL, United Kingdom.
| Text Revised: September 2016
1 The enclosed applicators (which include a pump spray, extended spray tip and dropper applicator) 1 ! are CE registered components (CE 0120) and are manufactured by Wrafton Laboratories Limited, i Braunton, Devon EX33 2DL UK.
| Q £0I20
! If you would like any further information about this product, please contact The Boots Company PLCl
| To request a copy of this leaflet in Braille, large print or audio please call, free of charge: i 0800 198 5000 (UK only) i Please be ready to give the following information:
This is a service provided by the Royal National Institute of Blind People.
ARTWORK ONLY
ARTWORK TEXT SIZE
|
Main Headings: |
12pt |
|
Sub Headings: |
lOpt |
|
Body Copy: |
apt |
|
PACK MOCK LIP | ||
|
Prod net Name: Boots Hairloss Treatment Regular Strength | ||
|
Product Licence No.: 12063/0038 Wordi ng Ref: WiRA approved v! dated 15/07/2015 (BTC189793 acton Cl | ||
|
Status: Pack Details: Pack Size: |
Internally approved HDPE bottle with 24mm polypropylene white cap and spray-pump/dropper applicator containing 60ml of solution. 60 ml | |
|
Version No. |
Date Issued |
Reason For Change |
|
1 |
10/06/2016 |
Clinical updates to the SPC with consequential updates to the Pit |
|
2 |
08/09/2016 |
RFI - Section 4 amended. |
|
3 |
29/09/2016 |
RFI - Further amends to Section 4. |
TECHNICAL 1MF0: PRINTER PLEASE NOTE Trident have created this artwork to a generic print process specification and repro has not been applied. Please call your Boots contact to discuss any amends or if anything falls beneath your print tolerances.
|
Trident Reference No: BTC233584 | |
|
Zen Ref: |
TR1138545 |
|
Category: |
Healthcare |
|
Sub-Category: |
Femcare |
|
Brand: |
Core |
|
Pack Type: |
leaflet |
|
Variant: |
Boots Hair Loss Treatment Regular |
|
Strength - Leaflet | |
|
Action: |
C |
|
Date: |
12/10/16 |
|
Country: |
UK |
|
Component Code: |
C6ZA3QAJ1 |
|
Item Code: |
34-G3-311 |
|
CAD Ref No: |
300x139.7mm - L160 |
|
Printer: |
N/A |
|
Substrate: |
White Paper |
|
Barcode Type: |
N/A |
|
Barcode Number: |
N/A |
|
Magnification: |
N/A |
|
Barcode Truncated By: N/A | |
|
(smallest bar) | |
|
Edgemark Position: |
N/A |
|
Pharmacode No/NE: |
1011110000(1775) |
Technical a Non Printing Items
| Cutter | Guide,

£3-
TRIDENT
Cormaught House, Connaught Road, Kingswood Business Park, Hull, Hl)7 3AP, England. T: +44 (0) 1482 828100
Please note that any low resolution paper Canon colour copies associated with this job should be referred to for content, layout and colour separation only.
UNDER NO CIRCUMSTANCES SHOULD THIS ARTWORK BE ALTERED WITHOUT PRIOR PERMISSION FROM TRIDENT.
STUDIO USE ONLY Michael Woodhead via
Sm.Art check results: G=1;0=0;R=0; - MW -12/10/1614:21 SS!

Information for the user

Hair Loss Treatment Regular Strength
(Minoxidil)
Read all of this leaflet carefully before you take this medicine because it contains Important Information you need to know.
This medicine is available without prescription, however, you still need to use this product carefully to get the best results from it. Keep this leaflet as you may need to read it again. Ask your pharmacist if you need more information or advice.
1. What is this medicine and what is it used for?
This medicine contains:
• minoxidil which acts on the scalp to help hair growth on the bald area,
This solution is used for the treatment of common hereditary baldness (a condition called alopecia androgenetica).
2. Is this medicine suitable for you?
Do not take this medicine if you:
• are allergic to minoxidil or any of the other ingredients
• suffer from high blood pressure, whether treated or not
• have scalp conditions, including psoriasis (an itchy inflammatory skin condition) or sunburn
• have a shaved scalp
• are using other medicines or occlusive dressings, such as plasters or air tight dressings, on your scalp
• are pregnant or breast feeding
• are younger than 18 years or older than 65 years.
If you are using other medicines that are applied to the scalp which contain any of the following ingredients, please see your doctor or pharmacist before taking this medicine:
• corticosteroids
• tretinoin
• dithranol
• petrolatum
• guanethidine.
These medicines may cause minoxidil to be absorbed quickly and increase the risk of side effects.
If you are taking any of the following medicines please see your doctor:
• medicines for high blood pressure that dilate (widen) the blood vessels, such as hydralazine.
Information about some of the ingredients in this medicine:
• Contains propylene glycol.
May cause skin irritation.
• Contains ethanol (alcohol).
Can cause burning and irritation to the eyes, if the product comes into contact with the eyes, broken skin or lining of the mouth, throat or nose, bathe the area with large amounts of cool tap water.
3. How to use this medicine
Before you start to use this medicine, check with your doctor that your scalp is normal and healthy. This medicine is for use on the scalp only. Do not apply it to other areas of the body. Make sure that the scalp is clean and dry before applying.
Men and women aged 18 to 65 years:
Apply 1 ml of the solution to the bald area twice daily, using the applicators provided (below).
Do not use more than 2 ml of the solution In 24 hours.
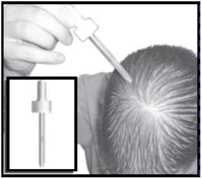
Pump spray applicator
Extended spray tip applicator

Dropper
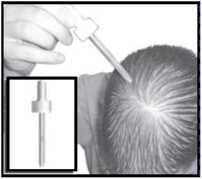
This is useful for large areas of baldness.
•spray several times away from your head and body prior to first application to remove any airlocks •aim the pump at the centre of the bald area
•press once and spread the solution with your fingertips over the whole area
•repeat for a total of 6 sprays to apply the 1 ml dose •avoid breathing the spray mist.
Do not exceed the stated dose.
If you use too much of the solution by mistake, or if it is accidentally swallowed:
Contact your doctor or pharmacist straight away.
If you use your fingertips to rub the solution into your scalp, make sure that you wash your hands afterwards.
This is useful for small areas, or under the hair,
•the pump spray applicator must be in place to use this applicator •attach the extended applicator to the spray applicator •use in the same way as the pump spray applicator.
This is useful for small areas, •fill the dropper to the 1 ml mark
•apply the solution to the bald area until the dropper is empty.
i If the product comes into contact with the eyes, broken skin or lining of i ! the mouth, throat or nose:
1 Bathe the area with large amounts of cool tap water. 1
i If no re-growth occurs after 12 months use, stop using this medicine. ■
! The hair may take up to 4 months to re-grow, and in some people may not grow at all. The new hair ! ; may be fine hair, similar to baby hair. This medicine is a treatment and needs to be continued for hair ] i growth to be maintained. !
| If you stop the treatment, the new hair will disappear after 3 to 4 months j
i 4. Possible side effects
i Most people do not have any side effects while taking this medicine. However, if you experience any i | of the following side effects, or anything else unusual happens, stop using the medicine immediately, [ i and see your doctor or pharmacist.
If any of the below effects occur, seek medical advice immediately:
[• swelling of the face, lips, tongue and/or throat j
;• palpitations and/or fast heart beat
'• changes such as skin rash and redness leading to itching, dry and flaking skin and/or swelling where1 | the product is applied, or around the ears and face, blistering, bleeding and ulcers chest pain i
[• swelling in the feet and/or legs
i* sudden, unexplained weight gain i
'• dizziness, feeling faint due to low blood pressure. 1
J If any of the below effects occur, discontinue use and seek advice from ; ; your pharmacist:
! Very common (may affect more than 1 in 10 people):
Common (may affect up to 1 in 10 people):
!• unwanted hair growth on the face or body \
'• itching [• rash
Uncommon (may affect up to 1 in 100 people):
■• feeling sick and/or being sick ■
[• flaking skin, skin rash, dry skin or other skin problems such as dermatitis
i» acne (spots and oily skin) i
'• changes in hair colour/texture of the newty grown hair !• temporary hair loss.
I Reporting of side effects
| If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side j i effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme i ' at: www.mhra.aov.uk/vellowcard. 1
| By reporting side effects you can help provide more information on the safety of this medicine.
i 5. How to store your medicine
; Keep out of the sight and reach of children.
i Do not use this medicine after the expiry date printed on the pack.
1 Store upright, below 25°C and protect from light. 1
| When the bottle is empty, the bottle and applicators must be disposed of safety. j
! 6. What is in this medicine?
i Each 1 ml dose of clear solution contains the active ingredient: minoxidil 20 mg i
! The other ingredients are: propylene glycol, ethanol, water.
| This pack contains 60 ml of solution and 3 applicators. j
i 7. Who makes this medicine?
i The Marketing Authorisation holder and manufacturer is Wrafton Laboratories Limited, Braunton, i | Devon, EX33 2DL, United Kingdom.
| Text Revised: September 2016
1 The enclosed applicators (which include a pump spray, extended spray tip and dropper applicator) 1 ! are CE registered components (CE 0120) and are manufactured by Wrafton Laboratories Limited, i Braunton, Devon EX33 2DL UK.
| Q £0I20
! If you would like any further information about this product, please contact The Boots Company PLCl
| To request a copy of this leaflet in Braille, large print or audio please call, free of charge: i 0800 198 5000 (UK only) i Please be ready to give the following information:
This is a service provided by the Royal National Institute of Blind People.
ARTWORK ONLY
ARTWORK TEXT SIZE
|
Main Headings: |
12pt |
|
Sub Headings: |
lOpt |
|
Body Copy: |
apt |
|
PACK MOCK LIP | ||
|
Prod net Name: Boots Hairloss Treatment Regular Strength | ||
|
Product Licence No.: 12063/0038 Wordi ng Ref: WtRA approved v! dated 15/07/2015 (BTC189793 act'on Cj | ||
|
Status: Pack Details: Pack Size: |
Internally approved HDPE bottle with 24mm polypropylene white cap and spray-pump/dropper applicator containing 60ml of solution. 60 ml | |
|
Version No. |
Date Issued |
Reason For Change |
|
1 |
10/06/2016 |
Clinical updates to the SPC with consequential updates to the Pit |
|
2 |
08/09/2016 |
RFI - Section 4 amended. |
|
3 |
29/09/2016 |
RFI - Further amends to Section 4. |
TECHNICAL 1MF0: PRINTER PLEASE NOTE Trident have created this artwork to a generic print process specification and repro has not been applied. Please call your Boots contact to discuss any amends or if anything falls beneath your print tolerances.
|
Category: Sub-Category: Brand: Pack Type: Variant: Action: Date: Country: |
Healthcare Femcare Core Leaflet Boots Hair Loss Treatment Regular Strength - Leaflet C 12/10/16 UK |
|
Component Code: |
C6ZA3QAJ1 |
|
Item Code: |
34-G3-311 |
|
CAD Ref No: Printer: |
300x139.7mm - L160 |
|
Substrate: |
White Paper |
|
Barcode Type: |
N/A |
|
Barcode Number: |
N/A |
|
Magnification: |
N/A |
|
Barcode Truncated By: N/A (smallest bar) | |
|
Edgemark Position: |
N/A |
|
Pharmacode No/NE: |
1011110000(1775) |
Technical a Non Printing Items
| Cutter | Guide,

STUDIO USE ONLY vl.0