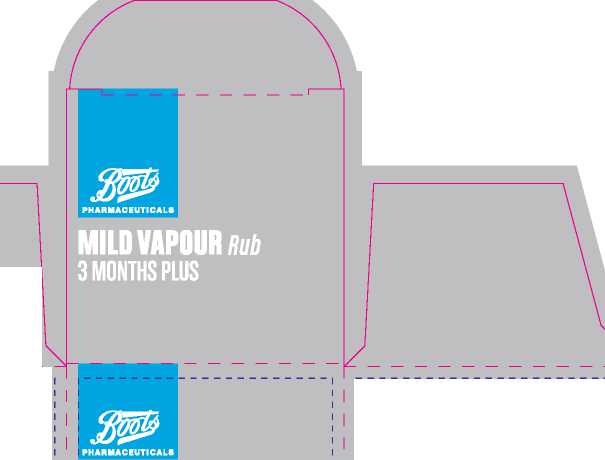
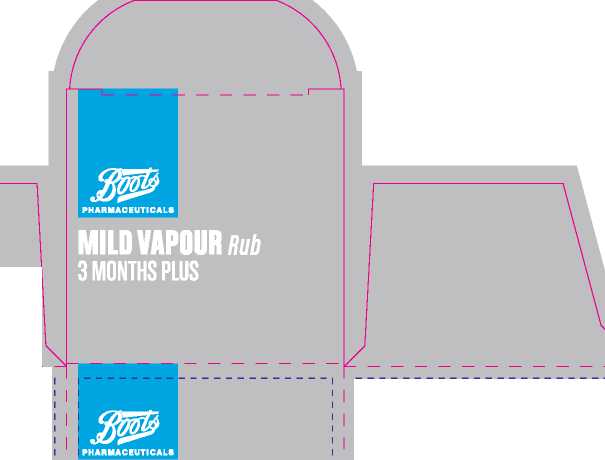
Boots Mild Vapour Rub 3 Months Plus
201 mm
190 mm
/How to use this medicine
Check the seal is not broken before first use, If it is, do not use the medicine.
|
m |
Howmuchtouse |
How often to use |
|
Children of 3 |
Apply a small amour! to |
When you need to |
|
months and |
the chest and back |
but particularly at |
|
over and |
(for older childien and adults |
bedtime |
|
adults |
use a moderate amount) |
Do not use on children under 3 months.
If your child's symptoms do not go away talk to your pharmacist or doctor.
! BeomeoneaccidentelyiwalowssonwGotoprnea
with p, Do not try to make them sick,
Possible side effects
Most people will not have problems, but some may get someoftnese:
• Occasionally, an allergic skin reaction (skin rash, red, itchy skin)
• Irritation ofthe skin
If p get any side effects, talk to pr doctor, pharmacist or nurse.This includes any possible side effects not listed on this carton. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.aowuk/vellowcard. By reporting side effects you can help provide more information on the safety| of this medicine.
How to store this medicine
Do not store above 25°C.
Keep al medches out ofthe sight ant teach of cNttn.
Use by the date on the side panel of the carton.
Active ingredients
This ointment contains Eucalyptus Oil 1.5% w/w, Levomenthol 1 % w/w, Racemic Camphor 6% w/w.
Also contains: white soft paraffin.
PL 00014/0562 Text prepared 02/15
Manufactured by BCM Ltd Nottingham NG2 3AA for the MAH: The Boots Company PLC Nottingham NG23AA
■)S!3euiiBL|d jnoA>|SB eojApBaioni pseu noAji
Eucalyptus, Levomenthol, Racemic Camphor
Read all of this carton for full instructions.
What this medicine is for
This medicine contains a combination of volatile oils for effective cold relief. It can be used to clear congestion, blocked noses and coughs due to colds.
Before you use this medicine
If yow child is uider 3 months If yov child is allergic to any of the ingredients Information for adults intending to use this medicine: All of the information on the carton applies to p as well. Talk to your pharmacist or doctor if you are pregnant or
V Clears congestion and stuffy noses
V Eases colds
V Helps breathing
tL*

45ge

Main Headings: 9pt
Sub Headings: 8pt
Body Copy: 7pt
|
PACK 1VIOCK UP | |||
|
Product Name: |
Boots Mild Vapour Rub 3 months plus | ||
|
Product Licence No.: |
00014/0562 | ||
|
Wording Ref: |
MHRA approved, V115/03/11 (BTC56771, action Cl | ||
|
Status: |
Internally approved | ||
|
Pack Details: |
Thermoset plastic cap or a polystyrene jar | ||
|
with an unlined polypropylene cap. | |||
|
Pack Size: |
45 g | ||
|
Version No. |
Date Issued |
Reason For Change | |
|
1 |
24/02/15 |
Add yellow card warning and change manufacturer to BCM Ltd NG2 3AA. Move Lot ft Use By for D10 on-line verification. | |
|
Trident Reference No: BTC177466 | |
|
Zen Ref: |
TR926342 |
|
Category: |
Healthcare |
|
Sub-Category: |
Childrens 0TC |
|
Brand: |
Core |
|
Pack Type: |
Carton |
|
Variant: |
Mild Vapour Chest Rub 3 months+ |
|
Action: |
B |
|
Date: |
02/03/15 |
|
Country: |
UK |
|
Component Code: |
LCVQ9 |
|
Item Code: |
23-16-471 |
|
CAD Ref No: |
47.5x47.5x67.5mm |
|
Printer: |
N/A |
|
Substrate: |
White Carton Board |
Barcode Type: EAN 13
Barcode Number: 5000167081435
Magnification: 100%
Barcode Truncated By: 4.5 mm
(smallest bar)
Edgemark Position: 1
Pharmacode No/NE: N/A
Technical Et Non Printing Items Cutter | Guides
Colours

190 mm
/How to use this medicine
Check the seal is not broken before first use, If it is, do not use the medicine.
|
m |
how much to use |
How often to use |
|
Children of 3 |
Apply a small amour! to |
When you need to |
|
months and |
the chest and back |
bPoaiticularlyat |
|
over and |
(for older chikfren and adults |
Mm» |
|
adults |
use a moderate amount) |
L___ |
Do not use o^ oh^ren under 3 months.
If your child's symptoms do not go away talk to your Pharmacia Oi doctor.
! lf(omeone»Xu«itaL, malms so,,«: Go,, hospital tarty department straight ary. Take this medicine with you Tonofytorrkf‘hr'sick, • «
• • • •
Possible side effects
Most people will not have problems, but some may get someoftnese:
• Occasionally, an allergic skin reaction (skin rash, red, itchy skin)
• Irritation ofthe skin
If you get any side effects, talk to your doctor, pharmacist or nurse.'lh1" includes any mssible side effects not listed on this carton. Yc i can also report swe '■'facts directly via the Yellow Card Scheme at: www.mhra.aov.uk/vellowcard. By reporting side effect yj (~n help pro.Je more information on the safety| of this medicine.*
How to store this reidne
Do not store above 25°C. > i
Keep al medches out of *te sight ant teach of cNttn.
Use by the date on the aide panel of the carton.
Active ingredients
This ointment contains Eucalyptus Oil 1.5% w/w, Levomeiijiol 1% w/w,. iacem«, CanviOi 6% w/w.
Also contain. white sc‘ paraffin.
PL0001./0J2 • • •••
Text prepared 02/15
Manufactured by BCM Ltd Nottingham NG2 3AA for the MAH: The Boots Company PLC Nottingham NG23AA
■)S!3euiiBL|d jnoA>|SB eojApBaioni pseu noAji
Eucalyptus, Levomenthol, Racemic Camphor
|
I |
V Clears congestion | |
|
I |
and stuffy noses V Eases colds | |
|
I |
V Helps breathing |
I/, * /M, |
|
I |
II * ~'\l |
Read all of this carton tor full instructions.
What this medicine is for
This medicine contains a combination of volatile oils for effective cold relief. It can be used to clear congestion, blocked noses and coughs due to colds.
Before you use this medicine
If yow child it inter 3 months If yov child is allergic to any of the ingredients Information for adults intending to use this medicine: All of the information on the carton applies to you as well. Talk to your pharmacist or dcxrtor if you are pregnant or

45ge
167
23-16471


Main Headings: 9pt
Sub Headings: 8pt
Body Copy: 7pt
|
PACK MOCK LIP | |||
|
Product Name: |
Boots Mild Vapour Rub 3 months plus | ||
|
Product Licence No.: |
00014/0562 | ||
|
Wording Ref: |
MHRA approved, V115/03/11 (BTC56771, action Cl | ||
|
Status: |
Internally approved | ||
|
Pack Details: |
Thermoset plastic cap or a polystyrene jar | ||
|
with an unlined polypropylene cap. | |||
|
Pack Size: |
45 g | ||
|
Version No. |
Date Issued |
Reason For Change | |
|
1 |
24/02/15 |
Add yellow card warning and change manufacturer to BCM Ltd NG2 3AA. Move Lot ft Use By for D10 on-line verification. | |
|
Trident Reference No: BTC177466 | |
|
Zen Ref: |
TR926342 |
|
Category: |
Healthcare |
|
Sub-Category: |
Childrens 0TC |
|
Brand: |
Core |
|
Pack Type: |
Carton |
|
Variant: |
Mild Vapour Chest Rub 3 months+ |
|
Action: |
B |
|
Date: |
02/03/15 |
|
Country: |
UK |
|
Component Code: |
LCVQ9 |
|
Item Code: |
23-16-471 |
|
CAD Ref No: |
47.5x47.5x67.5mm |
|
Printer: |
N/A |
|
Substrate: |
White Carton Board |
Barcode Type: EAN 13
Barcode Number: 5000167081435
Magnification: 100%
Barcode Truncated By: 4.5 mm
(smallest bar)
Edgemark Position: 1
Pharmacode No/NE: N/A
Technical Et Non Printing Items Cutter | Guides
Colours

190 mm

Main Headings: 9pt
Sub Headings: 8pt
Body Copy: 7pt
|
PACK MOCK LIP | |||
|
Product Name: |
Boots Mild Vapour Rub 3 months plus | ||
|
Product Licence No.: |
00014/0562 | ||
|
Wording Ref: |
MHRA approved, V115/03/11 (BTC56771, action Cl | ||
|
Status: |
Internally approved | ||
|
Pack Details: |
Thermoset plastic cap or a polystyrene jar | ||
|
with an unlined polypropylene cap. | |||
|
Pack Size: |
45 g | ||
|
Version No. |
Date Issued |
Reason For Change | |
|
1 |
24/02/15 |
Add yellow card warning and change manufacturer to BCM Ltd NG2 3AA. Move Lot ft Use By for D10 on-line verification. | |
|
Trident Reference No: BTC177466 | |
|
Zen Ref: |
TR926342 |
|
Category: |
Healthcare |
|
Sub-Category: |
Childrens 0TC |
|
Brand: |
Core |
|
Pack Type: |
Carton |
|
Variant: |
Mild Vapour Chest Rub 3 months+ |
|
Action: |
B |
|
Date: |
02/03/15 |
|
Country: |
UK |
|
Component Code: |
LCVQ9 |
|
Item Code: |
23-16-471 |
|
CAD Ref No: |
47.5x47.5x67.5mm |
|
Printer: |
N/A |
|
Substrate: |
White Carton Board |
Barcode Type: EAN 13
Barcode Number: 5000167081435
Magnification: 100%
Barcode Truncated By: 4.5 mm
(smallest bar)
Edgemark Position: 1
Pharmacode No/NE: N/A
Technical Et Non Printing Items Cutter | Guides
Colours

190 mm
Main Headings: 9pt
Sub Headings: 8pt
Body Copy: 7pt
|
PACK 1VIOCK UP | |||
|
Product Name: |
Boots Mild Vapour Rub 3 months plus | ||
|
Product Licence No.: |
00014/0562 | ||
|
Wording Ref: |
MHRA approved, V115/03/11 (BTC56771, action Cl | ||
|
Status: |
Internally approved | ||
|
Pack Details: |
Thermoset plastic cap or a polystyrene jar | ||
|
with an unlined polypropylene cap. | |||
|
Pack Size: |
45 g | ||
|
Version No. |
Date Issued |
Reason For Change | |
|
1 |
24/02/15 |
Add yellow card warning and change manufacturer to BCM Ltd NG2 3AA. Move Lot ft Use By for D10 on-line verification. | |
|
Trident Reference No: BTC177466 | |
|
Zen Ref: |
TR926342 |
|
Category: |
Healthcare |
|
Sub-Category: |
Childrens 0TC |
|
Brand: |
Core |
|
Pack Type: |
Carton |
|
Variant: |
Mild Vapour Chest Rub 3 months+ |
|
Action: |
B |
|
Date: |
02/03/15 |
|
Country: |
UK |
|
Component Code: |
LCVQ9 |
|
Item Code: |
23-16-471 |
|
CAD Ref No: |
47.5x47.5x67.5mm |
|
Printer: |
N/A |
|
Substrate: |
White Carton Board |
Barcode Type: EAN 13
Barcode Number: 5000167081435
Magnification: 100%
Barcode Truncated By: 4.5 mm
(smallest bar)
Edgemark Position: 1
Pharmacode No/NE: N/A
Technical Et Non Printing Items Cutter | Guides
Colours

28 mm
Read and keep carton for fidl mstructions.
/ How to use this medcine
Check that the seal is not broken before first use. For use on the skin only,
Do not store above 25°C.
Keep all medicines out of the sight and reach of children.
A
m
x
n
Children of 3 months and over and adults
|
How much louse |
How often to use |
|
Apply a smal amount to the chest and back (for older chidren and acLifta use a moderate amount) |
When yaj need to but particularly at bedtime |
Active ingredients: This ointment contains Eucalyptus Oil 1.5% w/w, Levomenthol 1% w/w, Racemic Camphor 6% w/w. Also contains: white soft paraffin.
PL 00014/0562 The Boots Company PLC Nottingham NG23AA

'.___JOoiiotjsfeOfichildren under Sjnojiths.^
|
Main Headings: |
5.5pt |
|
Sub Headings: |
5.5pt |
|
Body Copy: |
5.5pt |
Product Name:
Mild Vapour Rub 3 Months Plus
Product Licence No.: 00014/0562 Wording Ref: MHRA approved, v1 dated 15/03/11,
BTC56772 actionC
Status: Internally approved
Pack Details: Polystyrene jar with an uniined polypropylene
or thermoset plastic cap Pack Size: 45 g
Reason For Change
|
Trident Reference No: BTCl 77463 | |
|
Zen Ref: |
TR926331 |
|
Category: |
Healthcare |
|
Sub-Category: |
Childrens OTC |
|
Brand: |
Core |
|
Pack Type: |
Label |
|
Variant: |
Mild vapour rub 3 months plus |
|
Action: |
c |
|
Date: |
05/03/15 |
|
Country: |
UK |
|
Component Code: |
KEHC5 |
|
Item Code: |
23-16-471 |
|
CAD Ref No: |
2Bx120mm |
|
Printer: |
N/A |
|
Substrate: |
White Paper Label |
|
Barcode Type: |
N/A |
|
Barcode Number: |
N/A |
|
Magnification: |
N/A |
|
Barcode Truncated By: (smallest bar) |
N/A |
|
Edgemark Position: |
N/A |
|
Pharmacode No/NE: |
N/A |
|
Technical Et Non Printing Items Cutter | Guides | |
|
Colours | |
|
Overall Gloss Vamisti | |
|
_5_ | |
|
PANTONE 2925 C |
Mirror Sheer Silver | |||
|
_3_ |
_4_ | |||
Read and keep carton for fidl mstructions.
/ How to use this medcine
Check that the seal is not broken before first use. For use on the skin only,
Do not store above 25°C.
Keep all medicines out of the sight and reach of children.
A
m
x
n
Children of 3 months and over and adults
|
How much louse |
How often to use |
|
Apply a smal amount to the chest and back (for older chidren and acLifta use a moderate amount) |
When yaj need to but particularly at bedtime |
Active ingredients: This ointment contains Eucalyptus Oil 1.5% w/w, Levomenthol 1% w/w, Racemic Camphor 6% w/w. Also contains: white soft paraffin.
PL 00014/0562 The Boots Company PLC Nottingham NG23AA

'.___JOoiiotjsfeOfichildren under ^roorrths.^
|
Main Headings: |
5.5pt |
|
Sub Headings: |
5.5pt |
|
Body Copy: |
5.5pt |
Product Name:
Mild Vapour Rub 3 Months Plus
Product Licence No.: 00014/0562 Wording Ref: MHRA approved, v1 dated 15/03/11,
BTC56772 actionC
Status: Internal ly a pproved
Pack Details: Polystyrene jar with an uniined polypropylene
or thermoset plastic cap Pack Size: 45 g
Version No.
Date Issued
Reason For Change
|
Category: |
Healthcare |
|
Sub-Category: |
Childrens OTC |
|
Brand: |
Core |
|
Pack Type: |
Label |
|
Variant: |
Mild vapour rub 3 months plus |
|
Action: |
c |
|
Date: |
05/03/15 |
|
Country: |
UK |
|
Component Code: |
KEHC5 |
|
Item Code: |
23-16-471 |
|
CAD Ref No: |
2Bx120mm |
|
Printer: | |
|
Substrate: |
White Paper Label |
|
Barcode Type: |
N/A |
|
Barcode Number: |
N/A |
|
Magnification: |
N/A |
|
Barcode Truncated By: |
N/A |
|
(smallest bar) | |
|
Edgemark Position: |
N/A |
|
Pharmacode No/NE: |
N/A |
|
Technical Et Non Printing Items | |
|
Cutter | Guides | |
|
Colours | |
|
Overall Gloss Vamisti | |
|
_5_ | |
|
PANTONE 2925 C |
Mirror Sheer Silver | |||
|
_3_ |
_4_ | |||
28 mm

|
Main Headings: |
5.5pt |
|
Sub Headings: |
5.5pt |
|
Body Copy: |
5.5pt |
Product Name:
Mild Vapour Rub 3 Months Plus
Product Licence No.: 00014/0562 Wording Ref: MHRA approved, v1 dated 15/03/11,
BTC56772 actionC
Status: Internally approved
Pack Details: Polystyrene jar with an uniined polypropylene
or thermoset plastic cap Pack Size: 45 g
Reason For Change
|
Trident Reference No: BTCl 77463 | |
|
Zen Ref: |
TR926331 |
|
Category: |
Healthcare |
|
Sub-Category: |
Childrens OTC |
|
Brand: |
Core |
|
Pack Type: |
Label |
|
Variant: |
Mild vapour rub 3 months plus |
|
Action: |
c |
|
Date: |
05/03/15 |
|
Country: |
UK |
|
Component Code: |
KEHC5 |
|
Item Code: |
23-16-471 |
|
CAD Ref No: |
2Bx120mm |
|
Printer: |
N/A |
|
Substrate: |
White Paper Label |
|
Barcode Type: |
N/A |
|
Barcode Number: |
N/A |
|
Magnification: |
N/A |
|
Barcode Truncated By: (smallest bar) |
N/A |
|
Edgemark Position: |
N/A |
|
Pharmacode No/NE: |
N/A |
|
Technical Et Non Printing Items Cutter | Guides | |
|
Colours | |
|
Overall Gloss Vamisti | |
|
_5_ | |
|
PANTONE 2925 C |
Mirror Sheer Silver | |||
|
_3_ |
_4_ | |||
05/03/15 D10 on line verification