Brabio 20Mg/Ml Solution For Injection Pre-Filled Syringe
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
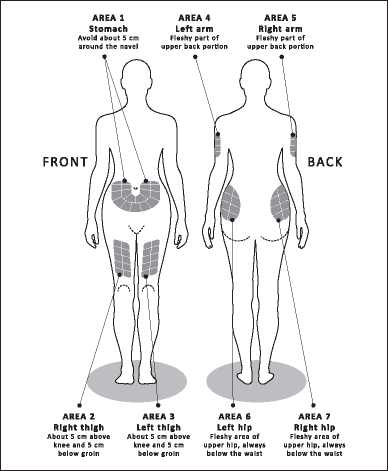
Figure 1
Package leaflet: Information for the user
Brabio 20 mg/ml solution for injection, pre-filled syringe
glatiramer acetate
What is in this leaflet
1. What Brabio is and what it is used for
2. What you need to know before you use Brabio
3. How to use Brabio
4. Possible side effects
5. How to store Brabio
6. Contents of the pack and other information
1. What Brabio is and what it is used for
Brabio is a medicinal product which modifies the way in which your body's immune system works (it is classed as an immunomodulating agent). The symptoms of multiple sclerosis (MS) are thought to be caused by a defect in the body's immune system. This produces patches of inflammation in the brain and spinal cord.
Brabio is used to reduce the number of times you suffer attacks of MS (relapses). It has not been demonstrated to help if you have any form of MS which does not have relapses, or hardly any relapses. Brabio may not have any effect on the length of time an MS attack lasts, or how badly you suffer during an attack.
It is used to treat patients who are able to walk without help.
Brabio may also be used in patients who have experienced symptoms for the first time which indicate a high risk of developing MS. Your doctor will rule out any other reasons which could explain these symptoms before you are treated.
2. What you need to know before you use Brabio
Do not use Brabio
• If you are allergic to glatiramer acetate or any of the other ingredients of this medicine (listed in section 6).
• If you are pregnant.
Warnings and precautions
Talk to your doctor or pharmacist before using Brabio
• if you have any kidney or heart problems as you may need to have regular tests and check-ups.
Children
Brabio is not to be used in children below the age of 12 years.
Elderly
Brabio has not been specifically studied in the elderly. Please ask your doctor for advice.
Other medicines and Brabio
Tell your doctor or pharmacist if you are
taking, have recently taken or might take any other medicines.
Pregnancy and breast-feeding Do not use Brabio if you are pregnant.
You should use effective birth-control (e.g. the 'pill' or condoms) to avoid pregnancy during the treatment with Brabio.
In case you would like to breast-feed while using Brabio, please discuss this first with your doctor.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Brabio is not known to influence the ability to drive or operate machinery.
3. How to use Brabio
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
The daily dose in adults and adolescents aged 12 years and over is one prefilled syringe (20 mg of glatiramer acetate), administered under the skin (subcutaneously).
It is very important to inject Brabio properly:
• Into the tissue under the skin (subcutaneous tissue) only (see "Instructions for Use" below).
• At the dose instructed by your doctor. Take only the dose prescribed by your doctor.
• Never use the same syringe more than once. Any unused product or waste must be discarded.
• Do not mix or co-administer the content of Brabio pre-filled syringes with
any product.
• If the solution contains particles, do not use it. Use a new syringe.
The first time you use Brabio you will be given full instructions and will be supervised by a doctor or nurse. They will be with you while you give yourself the injection and for half an hour afterwards, just to make sure you do not have any problems.
Instructions for use
Read these instructions carefully before using Brabio.
Before the injection, make sure you have everything you need:
• One blister with one Brabio pre-filled syringe
• Disposal unit for used needles and syringes.
• For each injection, take only one blister with one pre-filled syringe from the package. Keep all remaining syringes in the box.
• If your syringe has been stored in the refrigerator, take the blister containing the syringe out at least 20 minutes before you will inject the medicine so that it warms up to room temperature.
Wash your hands thoroughly with soap and water.
If you wish to use the Brabio injection device to make your injection, please refer to the instructions for use provided with the Brabio injection device.
Choose the injection site, using the diagrams in Figure 1.
There are seven possible areas on your body for injection: arms, thighs, hips and stomach (belly). Within each injection area there are several injection sites. Choose a different site for the injection every day. This will reduce the likeliness of any irritation or pain at the site of the injection. Rotate the injection sites within an area.
Do not use the same site each time.
Please note: do not inject in any area that is painful or discoloured or where you feel firm knots or lumps.
You should consider having a planned schedule for rotating injection sites and making a note of it in a diary. There are some sites on your body that may be difficult for self-injection (like the back of your arm). If you want to use these, you may require assistance.
How to inject:
• Remove the syringe from its protective blister by peeling back the paper label.
• Remove the shield from the needle.
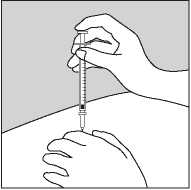
• Gently pinch up the skin with the thumb and forefinger of the free hand (Figure 2).
• Push the needle into the skin as shown in Figure 3.
• Inject the medicine by steadily pushing the plunger all the way down until the syringe is empty.
• Pull the syringe and needle straight out.
• Discard the syringe in a safe disposal container. Do not put used syringes into the household waste but dispose of them carefully in a puncture-proof container as recommended by your doctor or nurse.
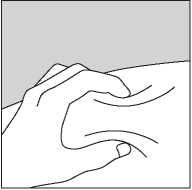
Figure 2 Figure 3
If you have the impression that the effect of Brabio is too strong or too weak, talk to your doctor.
If you use more Brabio than you should
Talk to your doctor immediately.
If you forget to use Brabio
Use it as soon as you remember but do not use a double dose to make up for forgotten individual doses. Use the next dose 24 hours later.
If you stop using Brabio
Do not stop using Brabio without consulting your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions (hypersensitivity)
You may rarely develop a serious allergic reaction to this medicine.
Stop using Brabio and contact your doctor immediately or go to the casualty department at your nearest hospital, if you notice any sign of these side effects:
• rash (red spots or nettle rash),
• swelling of the eyelids, face or lips,
• sudden shortness of breath,
• convulsions (fits),
• fainting.
Other reactions following Injection (Immediate Post-Injection reaction)
It's uncommon, but some people may get one or more of the following symptoms within minutes after injecting Brabio. They normally do not cause any problems and usually disappear within half an hour. However, if the following symptoms last longer than 30 minutes, contact your doctor immediately or go to the casualty department at your nearest hospital:
• flushing (reddening) of the chest or face (vasodilatation),
• shortness of breath (dyspnoea),
• chestpain
• pounding and rapid heartbeat (palpitations, tachycardia).
The following side effects have been reported with Brabio:
Very common side effects (may affect more than 1 in 10 people)
Infections, flu • anxiety, depression • headache • feeling sick • skin rash • pain in the joints or back • feeling weak, skin reactions at the injection site including reddening of skin, pain, formation of wheals, itching, tissue swelling, inflammation and hypersensitivity (these injection site reactions are not unusual and normally decrease over time), nonspecific pain
Common side effects (may affect up to 1 in 10 people)
Inflammation of the respiratory tract, gastric flu, cold sore, inflammation of the ears, runny nose, tooth abscess, vaginal thrush • non-malignant skin growth (non-malignant neoplasm of skin), tissue growth (neoplasm) • lymph node swelling
• allergic reactions • loss of appetite, weight gain • nervousness
• altered taste, increased tightness of muscle tone, migraine, speech disorder, fainting, tremor • double vision, eye disorder • ear disorder • cough, hay fever • disorder of anus or rectum, constipation, tooth decay, indigestion, difficulty in swallowing, bowel incontinence, vomiting
• abnormal liver function test • bruising, excessive sweating, itching, skin disorder, nettle rash • neck pain • urge to empty your bladder, frequent urination, inability to empty your bladder appropriately • chill, face swelling, wasting of tissue under the skin at injection site, local reaction, peripheral swelling due to build-up of fluid, fever
Uncommon side effects (may effect up to 1 in 100 people)
Abscess, inflammation of skin and the soft tissue underneath, boils, shingles, inflammation of kidney • skin cancer
• increased white blood cell count, reduced white blood cell count, spleen enlargement, low blood platelet count, change in form of white blood cells • enlarged thyroid, overactive thyroid • low alcohol tolerance, gout, increase
in blood fat levels, increase in blood sodium, decrease in serum ferritin • abnormal dreams, confusion, euphoric mood, seeing, hearing, smelling, tasting or feeling something that is not there (hallucinations), aggression, abnormal elevated mood, personality disorder, suicide attempt • hand numbness and pain (carpal tunnel syndrome), mental disorder, fits (convulsion), problems with handwriting and reading, muscle disorders, problems with movement, muscle spasm, nerve inflammation, abnormal nerve-muscle link leading to abnormal muscle function, involuntary rapid movement of the eyeballs, paralysis, foot drop (peroneal nerve palsy), unconscious state (stupor), visual blind spots • cataract, eye lesion in the cornea, dry eye, eye bleeding, droopy upper eyelid, pupil widening, wasting of the optic nerve leading to visual problems • extra heart beats, slow heart beats, episodic fast heart beats • varicose vein • periodic stops in breathing, nose bleeding, abnormally fast or deep breathing (hyperventilation), tight feeling in the throat, lung disorder, inability to breath due to throat tightness (choking sensation) • bowel inflammation, polyps in the colon, intestine inflammation, burping, ulcer in the gullet, inflammation of the gums, rectal bleeding, enlarged salivary glands • gallstones, liver enlargement
• swelling of the skin and soft tissues, skin contact rash, painful red skin lumps, skin lumps • swelling, inflammation and pain of joints (arthritis or osteoarthritis), inflammmation and pain of fluid-sacs lining the joint (exist in some of the joints), flank pain, decrease in the mass of muscles
• blood in the urine, kidney stones, urinary tract disorder, urine abnormality
• abortion • breast swelling, difficulties getting an erection, fall down or slip out of the place of pelvic organs (pelvic prolapse), sustained erections, disorders of prostate, abnormal PAP smear test (Smear Cervix Abnormal), testes disorder, vaginal bleeding, vaginal disorder • cyst, hangover, low body temperature (hypothermia), non-specific inflammation, destruction
of tissue at the injection site, problems with mucous membranes • disorders after vaccination
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme, at www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Brabio
Keep this medicine out of the sight and reach of children.
Store in a refrigerator (2°C - 8°C).
Brabio pre-filled syringes can be kept for up to one month outside the refrigerator between 15°C and 25°C. You can do this only once. After one month any Brabio pre-filled syringes that have not been used and are still in their original packaging must be returned to the refrigerator.
Do not freeze.
Keep the pre-filled syringes in the outer carton in order to protect from light.
Do not use this medicine after the expiry date which is stated on the label and carton after 'EXP'
The first two digits indicate the month and the last four digits indicate the year. The expiry date refers to the last day of that month.
Dispose of any syringes that contain particles.
Do not throw away any medicine via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6 Contents of the pack and other information
What Brabio contains
• The active substance is glatiramer acetate. 1 ml solution for injection (the contents of one pre-filled syringe) contains 20 mg glatiramer acetate.
• The other excipients are mannitol and water for injections.
What Brabio looks like and contents of the pack
Brabio solution for injection in pre-filled syringe is a sterile, clear colourless to slightly yellow/brownish solution.
If the solution contains particles, throw it away and start again. Use a new syringe.
7 pre-filled syringes 28 pre-filled syringes 30 pre-filled syringes
90 (3x30) pre-filled syringes
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
Synthon BV
Microweg 22, 6545 CM Nijmegen,
The Netherlands
Manufacturer(s):
Synthon Hispania SL
Castello 1, Poligono Las Salinas, 08830
Sant Boi de Llobregat, Spain
Synthon BV
Microweg 22, 6545 CM Nijmegen, The Netherlands
This leaflet was last revised in 03/2016
822685