Bramitob 300Mg/4Ml Nebuliser Solution
TECHNICAL INFORMATION / INFORMAZIONI TECNICHE
TECHNICAL WORKING_
DRAFT 1 (Mac - ID cc )
08/07/2015
APPROVAL ARTWORK _
Date: Date: Date:
1) ARTWORK LAB : (tecnichal part) □ (signature)
2) ARTWORK LAB. HEAD □ (signature)
3) MANUFACTURING DEPT. : □ (signature)
4) I certify here by under my personal responsibility that the signed text is in full compliance with the official version approved by the relevant regulatory Authority and in agreement with all regulations in force in the country, where the product will be distributed.
□ Approved (OK for the print)
□ Request for new draft
JOB TITLE _
NAME (IN CAPITAL LETTERS): _
Signature_ Date:_
5) ARTWORK LAB □ (Final check) □ PDF OK per TVS (signature)
Date:
|
CHIESI FARMACEUTICI S.p.A. - Parma - Italia VIETATA LA MANOMISSIONE |
D.Q.O.C. - Ufficio Grafico Autore: RB/ChB mail: r.boselli@chiesi.com | ||
|
(1) DESCRIZIONE MATERIALE F.E. BRAMITOB 300mg 4ml sol. da neb. CH. LTD | |||
|
(2) F.TO A.A. DIMENSIONI FOGLIO STESO DIMENSIONI FOGLIO FINITO PIEGATO F049 150 x 500 mm 150 x 62,5 mm |
(4) CODICE (8) DATA A.A. 87P48.06/01 09/06/2015 | ||
|
(3) N° PHARMACODE 172 (COD. A BARRE) |
TOT. PAG. PDF: N.2 |
(5) SOST. COD. 87P48.05/01 | |
|
COLORI DI STAMPA N° 1 flNERO + retino 50% FUSTELLA |
FONTS: in tracciati CORPO: / | ||
|
(7) Rif. richiesta di codifica del 28/05/2015 - (6) Motivo: - Regolatorio: - Chiesi UK address change - Tecnico: modifica cod. Chiesi, cod. laetus, cod. UK |
REPARTO:/ | ||
|
BIANCA | |||
NOTE PER FORNITORE: Dopo piegatura deve risultare visibile su un lato il nome del prodotto e il codice testo e sull’altro lato il codice a barre
6) ARTW. DEVEL. MANAGER □ Final PDF Approved for Print (signature)
Date:
7) PDF TRANSFERRED IN TVS:
(signature)
Date:
! PACKAGE LEAFLET: INFORMATION FOR THE USER
i Bramitob 300mg/4ml Nebuliser Solution
Read all of this leaflet carefully before you start taking this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Bramitob is and what it is used for
2. Before you use Bramitob
3. How to use Bramitob
4. Possible side effects
5. How to store Bramitob
6. Further information
1. What Bramitob is and what it is used for
Bramitob contains tobramycin which is an antibiotic belonging to a family called the aminoglycosides. It fights infections caused by Pseudomonas aeruginosa.
Bramitob is used for treating chronic chest infections in patients with cystic fibrosis caused by Pseudomonas bacteria. It kills the bacteria and helps to improve your breathing. Pseudomonas is a very common bacterium that infects nearly all patients with cystic fibrosis at some time during their lives. Some people do not get this infection until later on in their lives while others get it very young. If infection is not properly controlled it will continue to damage the lungs causing further problems. As Bramitob is breathed-in the antibiotic, tobramycin, can get straight into your lungs to work against the bacteria causing the infection.
Bramitob is indicated only for patients aged 6 years and older.
To achieve the best results please make every effort to use your medicine as instructed.
2. Before you use Bramitob
• If you are allergic (hypersensitive) to tobramycin, any of the other ingredients of Bramitob or any other type of aminoglycoside antibiotic
• If you are taking any of the medicines listed in the section below, Taking other medicines
Take special care with Bramitob:
The tobramycin in Bramitob is one of a group of medicines that can occasionally cause hearing loss, dizziness and kidney damage (see also Section 4 on the back of the leaflet, Possible side effects). It is important that you tell your doctor if any of the following applies to you:
• If your chest becomes tight after using your Bramitob. Your doctor will supervise your first dose of Bramitob and check your lung function before and after dosing. If you are not already doing so, your doctor may ask you to use a bronchodilator, (e.g. salbutamol), before using Bramitob.
• If you have ever suffered from any neuromuscular disorders such as parkinsonism or other conditions characterised by muscle weakness, including myasthenia gravis.
• If you have ever experienced kidney problems in the past. Before you start to use Bramitob, your doctor may check that your kidneys are working properly by testing a blood or urine sample. Your doctor may re-check this regularly during treatment.
• If you have ever experienced in the past
- ringing in your ears
- any other problems with your hearing
- dizziness.
Your doctor may test your hearing before starting Bramitob or at any time during your Bramitob treatment.
• If you are currently coughing up blood in your sputum. Inhaling medicines may cause you to cough and your doctor may ask you to stop using Bramitob until little or no blood appears in your sputum.
• If you are concerned that your Bramitob is not as effective as it should be. Bacteria can sometimes develop resistance to antibiotic treatment.
Taking other medicines:
Before starting treatment, please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
• Do not use Bramitob if you are taking diuretics (water tablets) containing furosemide or ethacrynic acid, without discussing this with your doctor.
• Do not use Bramitob if you are taking urea or mannitol (these products are used to treat serious conditions in hospitalised patients).
• Some other medicines can sometimes harm the kidneys or hearing and this could be made worse by Bramitob treatment.
You may be receiving injections of tobramycin or other aminoglycosides as well as inhaling Bramitob. Such injections, which may increase the very low body levels of aminoglycoside caused by inhaling Bramitob, should be avoided when the following medicines are being taken:
• Amphotericin B, cefalotin, ciclosporin, tacrolimus, polymyxins
• Platinum compounds (for example, carboplatin and cisplatin)
• Anticholinesterases (for example, neostigmine and pyridostigmine), botulinum toxin If this applies to you, you should speak to your doctor.
Pregnancy and breast-feeding:
If you are pregnant or could become pregnant or you are breastfeeding, talk to your doctor before using Bramitob.
Driving and using machines:
Bramitob has minor influence on the ability to drive and use machines.
In rare cases Bramitob may make you feel dizzy. It is therefore possible that Bramitob could affect your ability to drive a car or operate machinery.
Always use Bramitob exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure. Instructions for using Bramitob are given after the dosage section.
Do not mix or dilute your Bramitob with any other medicine in your nebuliser.
If you are taking several different treatments for cystic fibrosis you should take them in the following order:
- bronchodilator (e.g. salbutamol), then
- chest physiotherapy, then
- other inhaled medicines, then
- Bramitob
Also check the order with your doctor.
Bramitob should be used with a clean, dry PARI LC PLUS or PARI LC SPRINT reusable nebuliser (for your own personal use only) and a suitable compressor. Ask your doctor or physiotherapist for advice on which compressor to use.)
The single-dose Bramitob container should be opened just before use. Any unused solution that is not immediately used should be discarded.
Dosage:
• The dose (one 4 ml container) is the same for all persons aged 6 years and older.
• Use two single-dose containers per day for 28 days. Inhale the contents of one container in the morning and one in the evening. There should be a 12 hour gap between the doses.
• You then have 28 days without taking your medicine before starting another 28-day treatment cycle again.
• It is important that you keep using the product twice each day during your 28 days on treatment and that you keep to the 28-day on/28-day off cycle. Keep taking Bramitob in this way until your doctor tells you to stop.
If you use more Bramitob than you should:
If you inhale too much Bramitob you may get a very hoarse voice. Make sure you tell your doctor as soon as possible.
If you forget to use Bramitob:
• If there are more than 6 hours before you are due to use your next dose (container), use Bramitob now.
• If there are less than 6 hours before you are due to use your next dose (container), miss out the forgotten dose (container).
Then continue with your next dose as normal.
TECHNICAL WORKING
VIETATA LA MANOMISSIONE
D.Q.O.C. - Ufficio Grafico Autore: RB/ChB mail: r.boselli@chiesi.com
DRAFT 1 (Mac - ID cc ) 08/07/2015
APPROVAL ARTWORK
(1) DESCRIZIONE MATERIALE
1) ARTWORK LAB : (tecnichal part)
2) ARTWORK LAB. HEAD
3) MANUFACTURING DEPT. :
□ (signature) _
□ (signature) _
□ (signature) _
Date
Date
Date
|
(2) F.TO A.A. |
DIMENSIONI FOGLIO STESO |
DIMENSIONI FOGLIO FINITO PIEGATO |
(4) CODICE |
(8) DATA A.A. | |
|
F049 |
150 x 500 mm |
150 x 62,5 mm |
87P48.06/OI |
09/06/2015 |
(3) N° PHARMACODE 172 (COD. A BARRE)
TOT. PAG. PDF: N.2
(5) SOST. COD. 87P48.05/01
4) I certify here by under my personal responsibility that the signed text is in full compliance with the official version approved by the relevant regulatory Authority and in agreement with all regulations in force in the country, where the product will be distributed.
□ Approved (OK for the print)
□ Re
JOB T
□ Request for new draft
B TITLE _
NAME (IN CAPITAL LETTERS): . Signature_
Date
FONTS: in tracciati CORPO: /
(7) Rif. richiesta di codifica del 28/05/2015 - (6) Motivo:
- Regolatorio: - Chiesi UK address change
- Tecnico: modifica cod. Chiesi, cod. laetus, cod. UK
REPARTO: /
5) ARTWORK LAB □ (Final check) □ PDF OK per TVS
(signature)
Date:
6) ARTW. DEVEL. MANAGER □ Final PDF Approved for Print (signature).
Date
NOTE PER FORNITORE: Dopo piegatura deve risultare visibile su un lato il nome del prodotto e il codice testo e sull’altro lato il codice a barre
7) PDF TRANSFERRED IN TVS:
(signature)
Date
Instructions for use:
Bramitob is intended for use in a nebuliser, do not use it in any other way.
1. Wash your heddo thoroughln with soap and water befare opening your single-dose container according to the following instructions.
2. Cend the sicgle-dose container bagkwards and forwards (Figure A).
3. Cerefully separate s new container from the strip, firstly from the top, then in the middle (Figure B), leavine thn rest in the foil envelope.
4. Open the single-dose containeh by rotating the flap as indicated by the arrow (Figure C).
5. Gently squeeze the contents of the container into the nebuliser chamber (oigure D).
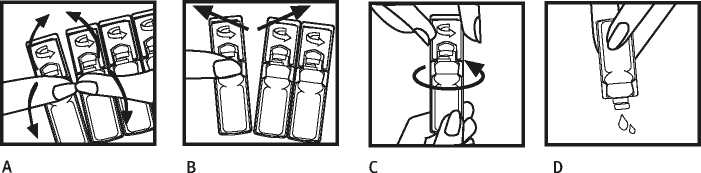
6. Turn on the compressor
7. Check if there is a pteady mist coming from the mouthpiece.
8. Sit or stand in an upright position so foal: you con breathe normally
9. Place the moufopiece between your teeth and on top of your tongue. Breathe normally, but only through your moufo (you may find noseclips helpful). Try not to block the end of the mouthpieca with your tongue.
10. Continue untN all fod Bramitob is used up, this: shionld tolue about 15 minntos.
11. If you are interrupted or need to cough or rest during your treatment:, turn off the compressor to save your medicine. Turn the compressor on again when you are ready to restart aour treatmqut.
If you have any further questions on the use of this product, ask your doctor or pharmacist. Looking after your nebuliser and compressor:
Please follow the manufacturer's instructions for the care and use of your nebuliser and compressor.
4. Possible side effects
Like all medicinee, Bramitob can cause side effects, although not everybody gets therm If yom ose not sure what thee side effects below are, aay your dmctor to explain them to you.
Thu most common side effects of Bramitob which may affect more than 1 in 100 people are: Cougm and hoarseness.
Uncommon siate effects of Bramitob which may affect: more than 1 in 1,000 people are: thrush in the mouth (candida infection), vertigo, loss of1 hearing, increased saliva quantities, inflammgtion o^lae tongue, rash, oore throat, hepatic enzymes increased in the blood, noisy breathihe, imusea, mucosal dry, coughing up blood, pain in the throat (oroplsarynmtis) or chest, loss of hearing, headache, shortnesa of lareath, weakness, producing more sputum (the substsnce you cough up) than normal, gastric pain and fungal lbfecrion.
Rere side effects which may affect more foan 1 in 10,000 people are: loss of appetite, ringing in the earv, nhest tighfoeds or bifficulty breathing, loss of voice, nose bleeds, venny nosa, mguth ulcers, vomiting taste disturbances, asthma, dizziness, loss of strength, fever, pain, laryngitis (voice alteration with sore foroat and difficulty swallow^).
Very Rare side effects which may affect less than 1 in 10,000 people are: swelling of lymph glaneo. droweiness, ear problems, ear pain, hyperventNation, sinusitis, diarrhoeai allergic reactions including urticaria and inuritus, deficiency offevaNable oxygen in the blood and bodily tissfes (hypoxia), back eaihi abdominal pain and generally feeling unwell.
If any of thy side effects geta serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Reporting of side effects
If you get any side effecte taH< to you! doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflel:. You can also report side effects directly via:
UKi Yellow Card Sahome at www.mhra.gov.ul</yellowcard.
ROI: HPRA Phafmacovigilance, Earlsfort Temace, IRL - Dyblih 2; Te.: +353 1 6764971; Fax: +353 1 6762517. Welcsite: www.hpra.ie, E-mail: medsafety@hpra.ie.
By reporting side effects you can help provide more information on the safety of this medicine.
5. Howto store Bramitob
• Keep out ou the reach and siget of chiloren
• For stegle use only. Do not use Bramitob ater foe expiry date which is stated uo the outer pack and label after EXP The expirb date refera to the last day of that month. You can use Brsmitob also if the colour of the solution varies
• In use shelf life: Bramitob bags (intact or opened) may be stored for up to 3 months at not more than 25°C.
• Store in a refrigerator (2-8°C). You can store the sinde-dose container for up to 3 months tit: not more than 25°C., if you don't have a refrigerator available and for traneporting purposesi
• Store your containers in the original packaging in order tea protect from light.
• After first openins the single-edse contdiner: Une immediatelya
• Aeer first use: Disnard thd used single-dose container immediate^
• Medicinhe should not be disposed of via wastewater or household waste. Ask your phaemacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Further information
What Bramitob contains:
3T0h0e macgt.ive substance is tobramycin. Each 4ml single-dose container contains tobramycin 300 mg.
The other ingredients are sodium chloride, sulphuric acid and sodium hydroxide (for pH adjustment), water for injections.
What framitob looks like and conthnts of the pack:
Bramitob appears as a clear yellowish solution.
Your Bramitod Nebulisbo Sofotisn ctmes in 4ml single-dose containers. There are 4 containers in each sealed bag, in box sizes of -4, 16, 28 or 56.
Not all pack sizes may be marketed.
IMarlceting Authorisation Holderand Manufacturer:
Madeting Authorisation Holder:
CNesi Limited, 333 Styal Road, Manchester, M22 5LG, United Kingdam.
Manufasturer: Chiesi Farmaceutid S.fxA, 26/A Via Palermo, 43122 Parma, Italy or Genetics S.p.A., Contradad Canfora, 84084 Fisciano (Italy)
This medicinal product is authorised in the Member States of the EEA under the following names:
|
Auktria: Bramitob |
Netherlandk: Bramitob |
|
Czech Ranyblic: mramitob |
Polefal Bramitob |
|
GermabB: Bramitob |
Portugal: Bhamitob |
|
Greece: Bramitob |
Slovak Republic: Bramitob |
|
Hungary: Bramitob |
Spain: Bramitob |
|
Irelayd: Bramitob |
United Kingdom: Bramitob |
|
Italy: Tobrineb |
Is this leaflet hard to see or read? Phone for help: 0161 488 5555 (from UK) +44161 488 5555 (from Ireland)
This leaflet was last approved in 07/2015 CPaaa5/9
PAG. 2/2