Bravelle 75 Iu Powder For Solution For Injection
PACKAGE LEAFLET: INFORMATION FOR THE USER
BRAVELLE
75 IU powder and solvent for solution for injection.
(Urofollitropin)
Read all of this leaflet carefully before you start using this
medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or nurse.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If you get any side effects, talk to your doctur or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What BRAVELLE is and what it is used for
2. What you need to know before you use BRAVELLE
3. How to use BRAVELLE
4. Possible side effects
5. How to store BRAVELLE
6. Contents of the pack and other information
1. Wh at BRAVELLE is and what it is used for
BRAVELLE is provided as a powder which must be mixed with liquid (solvent) before it is used. It is given as an injection under the skin.
BRAVELLE contains a hormone called follicle stimulating hormone (FSH). FSH is a natural hormone produced in both males and females. It helps the reproductive organs to work normally. The FSH in this medicine is obtained from the urine of postmenopausal women. It is highly purified, and is then known as urofollitropin.
BRAVELLE is used to treat female infertility in the following two situations:
i. Women who cannot become pregnant because their ovaries do not produce eggs (including polycystic ovarian disease). BRAVELLE is used in women who have already been given a medicine called clomiphene citrate to treat their infertility, but this medicine has not helped.
ii. Women in assisted reproduction programmes (including in vitro fertilisation/embryo transfer [IVF/ET], gamete intra-fallopian transfer [GIFT] and intracytoplasmic sperm injection [ICSI]). BRAVELLE helps the ovaries develop many egg sacs (follicles) where an egg might develop (multiple follicular development).
2. What you need to know before you use BRAVELLE
Before starting treatment with this medicine, you and your partner should be assessed by a doctor for causes of your fertility problems. In particular you should be checked for the following conditions so that any other appropriate treatment can be given:
• Underactive thyroid or adrenal glands
• High levels of a hormone called prolactin (hyperprolactinemia)
• Tumours of the pituitary gland (a gland located on the base of the brain)
• Tumours of the hypothalamus (an area located under the part of the brain called the thalamus)
If you know you have any of the conditions listed above, please tell your doctor before starting treatment with this medicine.
Do not use BRAVELLE:
• If you are allergic (hypersensitive) to urofollitropin or any of the other ingredients of this medicine (listed in section 6)
• If you have tumours of the uterus (womb), ovaries, breasts, pituitary gland or hypothalamus
• If you have cysts on your ovaries or enlarged ovaries (unless caused by polycystic ovarian disease)
• If you have malformations of the sexual organs which make a normal pregnancy impossible
• If you suffer from bleeding from the vagina where the cause is not known
• If you have fibroids of the uterus (womb) which make a normal pregnancy impossible
• If you are pregnant or breastfeeding
• If you have experienced an early menopause
Warnings and precautions
Talk to your doctor or nurse before using BRAVELLE.
If you get:
• Pain in the abdomen
• Swelling in the abdomen
• Nausea
• Vomiting
• Diarrhoea
• Weight gain
• Difficulty breathing
• Decreased urination.
Tell your doctor straight away, even if the symptoms develop some days after the last injection has been given. These can be signs of high levels of activity in the ovaries which might become severe.
If these symptoms become severe, the infertility treatment should be stopped and you should receive treatment in hospital.
Keeping to your recommended BRAVELLE dose and careful monitoring of your treatment will reduce your chances of getting these symptoms.
If you stop using this medicine you might still experience these symptoms. Please contact your doctor immediately if any of these symptoms occur.
While you are being treated with this medicine, your doctor will normally arrange for you to have ultrasound scans and sometimes blood tests to monitor your response to treatment.
Being treated with hormones like this medicine can increase the risk of:
• Ectopic pregnancy (pregnancy outside of the womb) if you have a history of fallopian tube disease
• Miscarriage
• Multiple pregnancy (twins, triplets, etc.)
• Congenital malformations (physical defects present in baby at birth).
Some women who have been given infertility treatment have developed tumours in the ovaries and other reproductive organs. It is not yet known if treatment with hormones like this medicine causes these problems.
Blood clots in the veins or arteries are more likely to occur in women who are pregnant. Infertility treatment can increase the chances of this happening, especially if you are overweight or if you or someone in your family (blood relative) has had blood clots. Tell your doctor if you think this applies to you.
Other medicines and BRAVELLE
Tell your doctor if you are taking, have recently taken or might take any other medicines.
Clomiphene citrate is another medicine used in the treatment of infertility. If BRAVELLE is used at the same time as clomiphene citrate the effect on the ovaries may be increased.
BRAVELLE can be used at the same time as MENOPUR. Please refer to section 3 ‘How to take BRAVELLE'.
Pregnancy and breast-feeding
This medicine should not be used during pregnancy or breast-feeding. Driving and using machines
This medicine is unlikely to affect your ability to drive and use machines.
Important information about some of the ingredients of BRAVELLE
BRAVELLE contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially ‘sodium-free'.
3. How to use BRAVELLE
Always use this medicine exactly as your doctor has told you. Check with your doctor if you are not sure.
i. Women who are not ovulating (not producing eggs):
Treatment should start within the first 7 days of the menstrual cycle (day 1 is the first day of your period). Treatment should be given every day for at least 7 days.
The starting dose is normally 75 IU daily (one vial of powder) but this may be adjusted depending on your response (up to a maximum of 225 IU - 3 vials of powders per day). A particular dose should be given for at least 7 days before the dose is changed. It is recommended that the dose should be increased by 37.5 IU (half a vial of powder) each time (and not more than 75 IU). The cycle of treatment should be abandoned if there is no response after 4 weeks.
When a good response is obtained a single injection of another hormone called human chorionic gonadotrophin (hCG), at a dose of 5,000 to
10.000 IU, should be given 1 day following the last BRAVELLE injection.
It is recommended to have sexual intercourse on the day of the hCG injection and the day after. Alternatively, artificial insemination (injection of sperm directly into the womb) may be performed. Your doctor will closely monitor your progress for at least 2 weeks after you have received the hCG injection.
Your doctor will monitor the effect of BRAVELLE treatment. Depending on your progress, your doctor may decide to stop treatment with BRAVELLE and not give you the hCG injection. In this case, you will be instructed to use a barrier method of contraception (e.g. condom) or not have sexual intercourse until your next period has started.
ii. Women in assisted reproduction programs:
If you are also receiving treatment with a GnRH agonist (a medicine which helps a hormone called Gonadotropin Releasing Hormone (GnRH) to work), BRAVELLE should be started approximately 2 weeks after the start of the GnRH agonist therapy.
In patients not receiving a GnRH agonist, BRAVELLE treatment should be started on day 2 or 3 of the menstrual cycle (day 1 is the first day of your period).
Treatment should be given every day for at least 5 days. The initial dose of this medicine is normally 150 - 225 IU (2 or 3 vials of powder). This dose may be increased according to your response to the treatment up to a maximum of 450 IU (6 vials of powder) per day. The dose should not be increased by more than 150 IU per adjustment. Normally treatment should not continue for more than 12 days.
If enough egg sacs are present, you will be given a single injection of a medicine called human chorionic gonadotrophin (hCG) at a dose of up to
10.000 IU to induce ovulation (release of an egg).
Your doctor will closely monitor your progress for at least 2 weeks after you have received the hCG injection.
Your doctor will monitor the effect of BRAVELLE treatment. Depending on your progress, your doctor may decide to stop treatment with BRAVELLE and not give you the hCG injection. In this case, you will be instructed to use a barrier method of contraception (e.g. condom) or not have sexual intercourse until your next period has started.
the following very common side effects may affect more than 10 of every 100 patients treated:
• Pain in the abdomen
• Headache
INSTRUCTIONS FOR USE
If your clinic has asked you to inject this medicine yourself, you should follow any instructions they provide.
The first injection of this medicine should be given under the supervision of a doctor.
DILUTING BRAVELLE:
This medicine is provided as a powder, and must be diluted before it is injected. The liquid which you should use to dilute this medicine is provided with the powder. This medicine should only be diluted immediately before use.
The following common side effects may affect between 1 and 10 of
every 100 patients treated:
To do this:
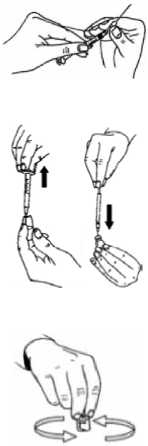
• Firmly attach the long, thick needle (drawing up /reconstitution needle) to the syringe.
• Break the top off the ampoule with the liquid.
• Draw up all of the liquid from the ampoule into the syringe.
• Insert the needle through the rubber top of the BRAVELLE powder vial and slowly inject all of the liquid. Aim at the side of the vial, to avoid creating bubbles.
• The powder should quickly dissolve (within 2 minutes) to form a clear solution. This normally happens when only few drops of solvent have been added.
• To help the powder dissolve, swirl the solution. Do not shake as this will cause air bubbles to form. If the solution is not clear or if it contains particles, it should not be used.
• Draw up the solution back into the syringe.
If you have been prescribed more than one vial of BRAVELLE powder per injection, you can draw up the solution (the first BRAVELLE dilution) back into the syringe and inject it into a second vial of powder. You can do this with up to six vials of powder in total - but only do as your doctor has told you.
If you have been prescribed MENOPUR at the same time as BRAVELLE, you may mix the two medicines by diluting BRAVELLE and injecting the solution into the MENOPUR powder. Allow this to dissolve, and draw up this combined solution: you can then inject them together instead of injecting each one separately.
INJECTING BRAVELLE:
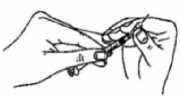
• Once you have your prescribed dose drawn up into the syringe, change the needle to the short, thin needle (the injection needle).
• Your doctor or nurse will tell you where to inject (e.g. front of the thigh, abdomen etc.)
• To inject, pinch the skin to produce a fold, and insert the needle in one swift motion at 90 degrees to the body. Press down on the plunger to inject the solution, and then remove the needle.
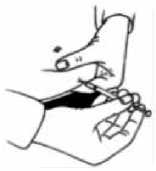
After removing the syringe, apply pressure to the injection site to stop any bleeding. Gently massaging the injection site will help to disperse the solution under the skin.
Do not put used items into normal domestic waste; these should be disposed of appropriately.
If you forget to use BRAVELLE
; Do not take a double dose to make up for a forgotten dose. Please tell a : nurse or doctor.
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Treatment with this medicine may cause high levels of activity in the ovaries, especially in women with polycystic ovaries. Symptoms include: pain in the abdomen, swelling in the abdomen, nausea, vomiting, diarrhoea, weight gain, difficulty breathing and decreased urination.
As complications to high levels of activity in the ovaries, blood clots and twisting of an ovary might occur. If you experience any of these symptoms contact your doctor immediately, even if they develop some days after the last injection has been given.
Allergic (hypersensitivity) reactions may occur when using this medicine. Symptoms of these reactions might include: rash, itching, swelling of the throat and difficulty breathing. If you experience any of these symptoms, contact your doctor immediately.
Urinary tract infection
Inflammation of the throat and nasal passage
Hot flushes
Nausea
Vomiting
Discomfort in the abdomen
Swelling in the abdomen
Diarrhoea
Constipation
Rash
Muscle spasms Pelvic pain
Overstimulation of the ovaries (high levels of activity)
Breast tenderness Vaginal bleeding Vaginal discharge Pain
Injection site pain and reactions (redness, bruising, swelling and/or itching)
Reporting of side effects
If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the Yellow Card Scheme, website: www.mhra.gov.uk/yellowcard .
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store BRAVELLE
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton. The expiry date refers to the last day of that month.
Do not store above 25°C. Do not freeze.
Store in the original container in order to protect from light.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to dispose of medicines you no longer required. These measures will help to protect the environment.
6. contents of the pack and other information
What BRAVELLE contains
The active substance is urofollitropin.
Each vial of powder contains 82.5 IU highly purified follicle stimulating hormone (FSH), urofollitropin. When reconstituted with the solvent provided, each vial delivers 75 IU of FSH.
The other ingredients in the powder are:
• Lactose monohydrate
• Sodium phosphate dibasic heptahydrate
• Polysorbate 20
• Phosphoric acid
• Water
The ingredients in the solvent are:
• Sodium chloride
• Water
• Hydrochloric acid
What BRAVELLE looks like and contents of the pack
This medicine is a powder and solvent for solution for injection.
The carton contains five or ten clear glass vials which contain a light powder. The carton also contains an equal number of clear glass ampoules containing a colourless solvent.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder of UK:
Ferring Pharmaceuticals Ltd, Drayton Hall, Church Road, West Drayton UB7 7PS.
PL 03194/0087 - Bravelle
PL 03194/0060 - Sodium Chloride Solution 0.9% w/v
Marketing Authorisation Holder of Ireland:
Ferring Ireland Ltd, United Drug House, Magna Drive, Magna Business Park, Citywest Road, Dublin 24 PA 1009/19/1
Manufacturers
Ferring GmbH
Wittland 11, D-24109 Kiel, Germany this leaflet was last revised in 04/2016

2009052201