Brevinor
Out of date information, search another5276
PACKAGE LEAFLET: INFORMATION FOR THE USER
BREVINOR®
0. 5 milligram (mg)/35 micrograms (gg) Tablets
norethisterone/ethinylestradiol
Important things that you SHOULD know about your medicine:
• Brevinor is an oral contraceptive medicine for use by women.
• This medicine has been prescribed for you. Do not pass it on to others.
• You should take Brevinor regularly as instructed by your doctor or nurse, in order for it to be effective. When taken as instructed, it is a very effective contraceptive. See Section 3 “What if I forget to take a tablet?”
• Most people do not have serious problems when taking Brevinor but side effects can occur - see Section 4 for details. If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor, nurse or pharmacist.
• Taking some other medicines may stop Brevinor from working properly. See Section 2 for details. Check with your doctor, nurse or pharmacist before taking any other medicines while you are taking Brevinor.
Please read the rest of this leaflet. It includes other important information on the safe and effective use of this medicine that might be especially important to you.
If you have any further questions, ask your doctor, nurse or pharmacist.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only.
Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Brevinor is and what it is used for
2. What you need to know before you take Brevinor
3. How to take Brevinor
4. Possible side effects
5. How to store Brevinor
6. Contents of the pack and other information
1. What Brevinor is and what it is used for
Brevinor is one of a group of medicines called combined oral contraceptives or “the Pill” for short.
Brevinor contains two hormones - a progestogen hormone called norethisterone and an oestrogen hormone called ethinylestradiol. These two hormones act together to prevent pregnancy from occurring.
2. What you need to know before you take Brevinor
^ Do not take Brevinor:
• if you are allergic to norethisterone, ethinylestradiol or any of the other ingredients of this medicine (listed in section 6).
• if you are currently pregnant.
• if you have had blood clots in the legs, blood clots in varicose veins, the lungs, the brain or elsewhere (coronary and cerebral thrombotic disorders).
• if you or a member of your family have ever had a problem with blood clots, including deep vein thrombosis (DVT).
• if you have had swelling (inflammation) of a vein caused by a blood clot.
• if you have had a heart attack or stroke or have had or have angina.
• if you have had or have high levels of fats in your blood (hyperlipidaemia) or other disorders of body fats.
• if you have had or have cancer of the breast, cervix, vagina or womb.
• if you have had the following during pregnancy:
• pruritus (itching of the whole body)
• jaundice (yellowing of the skin or eyes), for which your doctor could not find the cause
• pemphigoid gestationis (a rash previously known as herpes gestationis typically with blistering of the palms of the hands and the soles of the feet).
• if you have had or have severe chronic liver disease (liver tumours, Dubin-Johnson or Rotor syndrome).
• if you have had or have vaginal bleeding, for which your doctor could not find the cause.
• if you have had or have bad migraines.
^ Warnings and precautions
Talk to your doctor or pharmacist before taking Brevinor if you have any of the following conditions. This will help them decide if Brevinor is suitable for you:
• Migraine
• Headaches
• Slow or sudden development of visual disturbances such as complete or partial loss of vision
• Asthma
• Epilepsy (a condition where you suffer from fits)
• Diseases of the heart and blood vessels (cardiovascular disease)
• High blood pressure (hypertension)
• Kidney disease
• Diabetes
• Multiple sclerosis (a problem of the nervous system)
• Tetany (muscle twitches)
• Breast problems of any sort
• Varicose veins (widened or twisted vein usually in the leg)
• Liver dysfunction
• Severe depression
• Fibroids in your uterus
• Irregular periods
• Sharp pain in your abdomen
• Gallstones
• Sickle-cell anaemia
• Otosclerosis (an inherited form of deafness)
• Porphyria (a metabolic disease)
• Chloasma (brown patches on your skin which can happen during pregnancy but may not fade completely)
• Any disease that is likely to get worse during pregnancy.
Possible risk of Thrombosis (Blood Clot)
Some evidence suggests that women who take the Pill are more likely to develop various blood circulation disorders than women who don't take the Pill.
A thrombosis is a blood clot. A thrombosis can develop in veins or in arteries and can cause a blockage. The chance of a thrombosis forming in women taking the Pill and women not taking the Pill is rare. When blood clots form in arteries they can cause chest pain (angina), strokes (blood clots in or bleeding from the blood vessels in the brain) and heart attacks.
If blood clots form in veins they can often be treated, with no long-term danger. On rare occasions a piece of thrombosis may break off. It can travel to the lungs to cause a condition called pulmonary embolism. Therefore in rare cases a thrombosis can cause serious permanent disability or could even be fatal.
It is important to note that a thrombosis can form in people who are not taking the Pill as well as those who are taking it. The risk is higher in women who take the Pill than in women who don't take the Pill, but is not as high as the risk during pregnancy. The excess risk of thrombosis is highest during the first year a woman ever uses a combined oral contraceptive pill.
For healthy non-pregnant women: the chance of having a blood clot is about 5 in 100,000 each year.
For women taking the Pill containing either levonorgestrel or norethisterone (a second generation Pill): the chance of having a blood clot is about 15 in 100,000 each year.
For women taking the Pill containing desogestrel or gestodene (a third generation Pill): the chance of having a blood clot is about 25 in 100,000 each year.
For women who are pregnant: the chance of having a blood clot is about 60 in 100,000 pregnancies.
The risk of heart attacks and strokes for women who use the combined Pill increases with age and smoking. Other conditions also increase the risk of blood clots in the arteries. These include being greatly overweight, having diseased arteries (atherosclerosis), high blood pressure during pregnancy (pre-eclamptic toxaemia), high blood levels of cholesterol, and diabetes. If you have any of these conditions, you should check with your doctor or nurse to see if the Pill is suitable for you. Smokers over 35 are usually told to stop taking these pills.
Possible risk of Breast Cancer
Every woman is at risk of breast cancer whether or not she takes the Pill. Breast cancer is rare under the age of 40 years, but the risk increases as a woman gets older. Breast cancer has been found slightly more often in women who take the Pill than in women of the same age who do not take the Pill. If women stop taking the Pill, this reduces the risk so that 10 years after stopping the Pill, the risk of finding breast cancer is the same as for women who have never taken the Pill. Breast cancer seems less likely to have spread when found in women who take the Pill than in women who do not take the Pill.
It is not certain whether the Pill causes the increased risk of breast cancer. It may be that women taking the Pill are examined more often, so that breast cancer is noticed earlier. The risk of finding breast cancer is not affected by how long a woman takes the Pill but by the age at which she stops. This is because the risk of breast cancer strongly increases as a woman gets older.
The chart below shows the background chances of breast cancer at various ages for 10,000 women who have never taken the Pill (black bars) and for 10,000 women whilst taking the Pill and during the 10 years after stopping it (orange bars). The small extra risk of finding breast cancer can be seen for each age group. This small possible additional risk in women who take the Pill has to be balanced against the fact that the Pill is a very effective contraceptive and it helps prevent cancer of the womb or ovary.
Estimated number of breast cancers found in 10,000 women who took the Pill for 5 years then stopped, or who never took the Pill
5276
• There have been some reports on the risk of liver tumors and cervical cancer associated with the use of oral contraceptives.
• There is evidence to suggest that the use of combined oral contraceptives offers protection
-against both ovarian and endometrial cancer.
Cervical cancer
Some research suggests an increased risk of getting cancer of the cervix (neck of the uterus or womb) in women who take combined oral contraceptives for a long time. However, this may be due to other causes, such as sexual behaviour.
Liver cancer
Very rarely, tumours of the liver have been seen in women taking combined oral contraceptives, especially if they have been taken for a long time.
If you are worried about any of these things or if you have had cancer in the past, talk to your doctor to see if you should take the combined oral contraceptive pill.
Endometrial and ovarian cancer
Research shows that combined oral contraceptives protect against cancer of the ovary and cancer of the endometrium (lining of the womb).
If you are going to have a major operation
Make sure your doctor knows about it. You may need to stop taking Brevinor about 4 weeks before the operation until at least 2 weeks after the operation and until you are fully mobile. Alternatively, your doctor may prescribe an estrogen-free hormonal contraceptive.
Your doctor or nurse will advise whether you can still take Brevinor.
Medical check-ups
Your doctor or nurse will give you regular checkups while you are taking Brevinor. Your blood pressure will be checked before you start Brevinor and then at regular intervals whilst you are on Brevinor. You may be required to have an examination of your breasts, abdomen and pelvis including a cervical smear test at regular intervals, if this is considered necessary by the doctor.
Sexually transmitted diseases
Brevinor helps to prevent pregnancy. It will not protect against sexually transmitted diseases including AIDS. For safer sex, use a condom as well as your usual contraceptive.
^ Other medicines and Brevinor
Tell your doctor or pharmacists if you are taking, have recently taken, or might take any other medicines. This includes the following medicines, as the effect of Brevinor may be altered when they are taken at the same time:
• the herbal remedy St John's wort - Latin name Hypericum perforatum (depression)
• carbamazepine (epilepsy)
• oxacarbazepine (epilepsy)
• phenytoin (epilepsy)
• phenobarbital (sleeplessness, anxiety, epilepsy)
• primidone (epilepsy)
• topiramate (epilepsy)
• nelfinavir (HIV - Human Immunodeficiency Virus -infection)
• nevirapine (HIV infection and AIDS)
• ritonavir (HIV infection and AIDS)
• rifabutin (bacterial infection)
• rifampicin (bacterial infection)
• griseofulvin (fungal infection)
• modafinil (narcolepsy i.e. daytime sleepiness).
If you do need to take any of the medicines listed above, Brevinor may not be suitable for you. Your doctor or nurse will advise you whether to stop taking these medicines or use an additional contraceptive method, such as a condom whilst taking Brevinor.
^ Laboratory Tests
Brevinor may interfere with some tests, tell your doctor or nurse if you need to give samples for laboratory assessment.
^ Taking Brevinor with food and drink
Please refer to section 3.
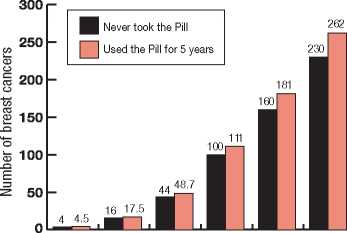
Took the P ill at
these ages:-Under 20 20-24 25-29 30-34 35-39 40-44
Cancers found up
to the age of: 30 35 40 45 50 55
Crease
Pregnancy, breast-feeding and fertility
Do not take Brevinor if you are pregnant or trying to become pregnant.
Do not take Brevinor if you are breast-feeding.
If you miss a period while you are taking Brevinor, tell your doctor, nurse or pharmacist. Your doctor, nurse or pharmacist will inform you about the increased risk to the foetus if you have become pregnant while taking Brevinor. You will need to have a pregnancy test before you continue to take Brevinor.
Driving and using machines
Brevinor is not known to affect the ability to drive or use machinery.
Brevinor contains lactose
Lactose is a type of sugar. If you suffer from diabetes or you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine.
3. How to take Brevinor
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Brevinor can be taken with or without food.
Starting your first blister strip
• Take the first tablet on your first day of bleeding.
This is the day when your period starts. If you are not having periods, ask your doctor or clinic when you should start taking your tablets.
• Take the tablet marked with the correct day of the week.
• You will be protected at once as long as you take a tablet every day.
• You can take the tablet at a time that suits you, but you must take it at about the same time every day.
• Take a tablet every day until you finish a blister strip.
• If you cannot start the tablet on the first day of your period you may start to take it on any day up to the fifth day. However, if you do this, you may not be protected for the first seven days, so you should use another method of contraception such as a condom during those days.
Starting the next blister strip
• Once you have finished all 21 tablets, stop for seven days. You will probably bleed during some or all of these seven days.
• Then, start the next blister strip. Do this whether or not you are still bleeding. You will always start the next blister strip on the same day of the week.
• You are protected during the seven day break, but only if you start the next blister strip on time. The first tablet in your blister strip is the worst pill of all to miss or take late.
^ If you notice a change in your periods
It is normal that your periods may become irregular and you may notice some bleeding between periods. Your periods may become lighter and you may occasionally have no bleeding during the tablet free days. Make a note of what happens so that you can tell your doctor or nurse at your next check-up.
^ If you take more Brevinor than you should
Taking too many tablets at once may make you sick, cause vaginal bleeding or breast swelling. Contact your doctor or go to your nearest hospital casualty department immediately.
^ If you forget to take Brevinor
• If you forget to take a tablet take it as soon as you remember and take the next one at your normal time. This may mean taking two tablets on the same day.
• If you are 12 or more hours late in taking one or more tablets, it may not work. As soon as you remember, take your last missed tablet and carry on taking them normally. However, you may not be protected for the next seven days, so either avoid sexual intercourse or use an extra contraceptive method, such as a condom.
• If you have fewer than seven tablets in your blister strip after you have missed taking a dose, you should complete the blister strip and start the next blister strip without a break. This will give you protection from when you took the last missed tablet. You may not have a period until the end of two blister strips, but this will not harm you. You may also have some bleeding on days when you take the tablets.
^ If you want to stop taking Brevinor or want to have a baby?
If you stop taking Brevinor, this will result in the loss of contraceptive protection and the risk of pregnancy.
If you wish to become pregnant, you should contact your doctor or nurse about stopping the tablets. It is advisable to stop taking Brevinor 3 months before you want to start trying to have a baby.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
^ If you change brands of oral contraceptive
• Take the first tablet of your new blister strip on the day immediately after you have finished your old blister strip. Your period will usually be delayed until the new blister strip is finished, but you may have some break-through bleeding during the first few days of the new blister strip. This is quite normal and you will still be protected against pregnancy.
^ If you have a stomach upset or you are sick
• Brevinor may not work if you are sick or have severe diarrhoea. You should carry on taking the tablets as normal, but use a condom while you are ill and for the next seven days. If these seven days run beyond the end of the blister strip, start the next pack without a break.
• If you do have a break, ask your doctor or nurse whether you need an extra contraceptive method, such as a condom.
^ If you have just had a baby
• If you are breast feeding, you should not take the combined oral contraceptive. This is because the oestrogen in the tablets may reduce the amount of milk you produce. You should be able to take another type of contraceptive instead. Ask your doctor or nurse for advice.
• If you are not breast feeding, you may start taking Brevinor 21 days after your baby is born. This will protect you immediately. If you start later than this, you may not be protected until you have taken the tablets for seven days.
^ If you have just had a miscarriage or abortion
You may be able to start taking Brevinor immediately. If you can, you will be protected straight away. Ask your doctor or nurse if you should do so.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects although not everybody gets them.
Tell your doctor or call an ambulance immediately if
you experience any of the following symptoms of an allergic reaction after taking this medicine. Although they are rare, the symptoms can be severe and you may need urgent medical attention or hospitalisation.
• Sudden wheeziness, difficulty in breathing, chest pain, fever, sudden swellings, rash or itching (especially affecting the whole body).
Stop taking Brevinor and contact your doctor straight away if you notice any of the following serious side effects. These may be signs of a blood clot.
• you are coughing up blood
• you have swelling or tenderness in your stomach
• you have a sudden sharp or severe pain in the chest
• you suddenly become short of breath or find breathing is painful
• you have painful or inflamed veins in your legs
• you have a first attack of migraine (a bad headache with sickness)
• you have migraines which get worse, especially if your sight is affected, you see flashing lights, your limbs feel weak, you lose the sensation or feel a different sensation in your limbs, or you have a fit
• you have sudden and unusual severe headaches
• you experience dizziness or you faint
• you develop a problem with your sight or speech.
Other side effects Brevinor may cause are:
• feeling sick
• stomach upset
• weight gain
• changes in appetite
• changes in the way your body breaks down sugars, fats or vitamins
• headache
• high blood pressure
• depression
• swollen or sore breasts
• change in sex drive
• worsening of womb disorders
• irregular vaginal bleeding.
Taking any medicine carries some risk. You can use the information in this leaflet, and the advice your doctor or nurse has provided you to weigh up the risks and benefits of taking the Pill. Don't be embarrassed, ask as many questions as you need to.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Brevinor
Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date which is stated on the carton and blister strip after EXP. The expiry date refers to the last day of that month.
Do not store above 25°C. Keep the blister in the outer carton in order to protect from light.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Brevinor contains
The active substances are norethisterone and ethinylestradiol.
Each Brevinor tablet contains 500 micrograms of norethisterone and 35 micrograms of ethinylestradiol The other ingredients are maize starch, polyvidone, magnesium stearate, lactose (see section 2 Brevinor contains lactose) and colouring E132.
What Brevinor looks like and contents of the pack
Brevinor tablets are blue and have the word ‘SEARLE' on one side and ‘BX' on the other side. They are packed in blister strips of 21 tablets and come in cartons containing either 21 or 63 tablets. Not all pack sizes maybe marketed.
Marketing Authorisation Holder and Manufacturer Marketing Authorisation Holder
Pfizer Limited Ramsgate Road Sandwich Kent
CT13 9NJ UK
Manufacturer
Piramal Healthcare UK Limited
Morpeth
Northumberland
NE61 3YA
UK
This leaflet was last revised in 08/2014.
Ref: BR 9_1
Crease
20715623