Bricanyl 0.5Mg-Dose Turbohaler



PATIENT INFORMATION LEAFLET
Bricanyl® 0.5mg/dose Turbohaler® (terbutaline sulphate)
Your medicine is known as above name but will be referred to as Bricanyl Turbohaler throughout this leaflet.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Bricanyl Turbohaler is and what it is used for
2. What you need to know before you use Bricanyl Turbohaler
3. How to use Bricanyl Turbohaler
4. Possible side effects
5. How to store Bricanyl Turbohaler
6. Contents of the pack and other information
1. What Bricanyl Turbohaler is and what it is used for
Bricanyl Turbohaler is an inhaler. It contains a medicine called terbutaline. This belongs to a group of medicines called ‘beta-agonists'. These work by relaxing certain muscles and opening up the airways in the lungs.
Bricanyl Turbohaler is used for asthma and other breathing problems where you have a tight chest and difficulty breathing.
2. What you need to know before you use Bricanyl Turbohaler
Do not use Bricanyl Turbohaler:
• If you are allergic to terbutaline.
Warnings and precautions
Talk to your doctor or pharmacist before using Bricanyl Turbohaler if: • You have diabetes. If so, you may need some extra blood sugar tests when you start using Bricanyl Turbohaler.
• You have a history of heart disease, irregular heart rhythm or angina.
• You have an overactive thyroid gland.
If you are not sure if any of the above apply to you, talk to your doctor or pharmacist before using Bricanyl Turbohaler.
Other medicines and Bricanyl Turbohaler
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. This includes medicines that you buy without a prescription and herbal medicines. Bricanyl can affect the way that some medicines work and some medicines can have an effect on Bricanyl.
In particular, tell your doctor or pharmacist if you are taking any of the following medicines:
• Steroid medicines (such as prednisolone).
• Medicines called ‘xanthines' (such as theophylline).
• Medicines called ‘beta-blockers' (such as atenolol or propranolol) including eye drops (such as timolol).
• Water tablets (diuretics) such as furosemide (also known as frusemide).
If you are to undergo surgery with general anaesthetics it is important that you inform your doctor about all medicines you use, including Bricanyl to protect you from adverse effects (e.g. irregular heart beat).
Pregnancy, breast-feeding and fertility
• If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
• If you become pregnant while you are using Bricanyl Turbohaler, talk to your doctor straight away.
Driving and using machines
Bricanyl is not likely to affect you being able to drive or use any machines.
3. How to use Bricanyl Turbohaler
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure. If Bricanyl Turbohaler is to be used by a child, make sure that they use it correctly.
How much to take
• The recommended dose is one inhalation as required.
• Do not take more than four inhalations in any 24 hour period.
• One inhalation from your Bricanyl Turbohaler should last for up to six hours.
Talk to your doctor straight away if:
• Your breathing is getting worse.
• You often wake at night with asthma.
• You start getting a tight chest.
• You are not getting relief from your current dose.
These are signs that your asthma is not being controlled. You may need a different or additional treatment straight away.
Please read the complete instructions carefully before you start to take your medication
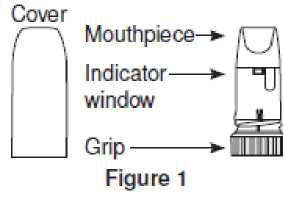
Turbohaler is a multidose inhaler from which very small amounts of powder are administered (Figure 1).
When you breathe in through Turbohaler the powder is delivered to your lungs. It is therefore important that you
inhale forcefully and deeply
through the mouthpiece.
How to prepare a new Turbohaler for use
Before using Turbohaler for the first time you need to prepare the inhaler for use.
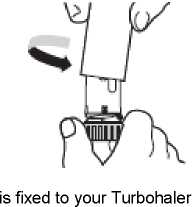
Unscrew and lift off the white cover. Hold the Turbohaler upright with the blue grip downwards (Figure 2). Do not hold the mouthpiece when you turn the blue grip. Turn the blue grip as far as it will go in one direction, and then back again in the opposite direction as far as it will go.
It does not matter which way you turn first.
During this procedure you will hear a click.
Perform the procedure twice.
The turbohaler is now prepared for use, and you should not repeat the above procedure again. To take a dose, please continue according to the instructions below.
How to take an inhalation
Every time you need to take an inhalation, follow the instructions below.
1. Unscrew the white cover and lift it off.
2. Hold your Turbohaler upright with the blue grip at the bottom.
3. Do not hold the mouthpiece when you load your Turbohaler. To load your Turbohaler with a dose, turn the blue grip as far as it will go in one direction. Then turn it as far as it will go in the other direction (it does not matter which way you turn it first). You should hear a click sound. Your Turbohaler is now loaded and ready to use. Only load your Turbohaler when you need to use it.
4. Hold your Turbohaler away from your mouth. Breathe out gently (as far as is comfortable). Do not breathe out through your Turbohaler.
5. Place the mouthpiece gently between your teeth.
Close your lips. Breathe in as deeply and as hard as you can through your mouth. Do not chew or bite on the mouthpiece.
6. Remove your Turbohaler from your mouth. Then breathe out gently. The amount of medicine that is inhaled is very small. This means you may not be able to taste it after inhalation. If you have followed the instructions, you can still be confident that you have inhaled the dose and the medicine is now in your lungs.
7. If you are to take a second inhalation, repeat steps 2 to 6.
8. Replace the cover by screwing it back on tightly.
With each inhalation some medication may stick to the inside of your mouth and throat. To reduce the risk of side effects it is advised that you, when possible, rinse your mouth with water after using Bricanyl Turbohaler.
Do not try to remove or twist the mouthpiece. It and must not be taken off. Do not use your Turbohaler if it has been damaged or if the mouthpiece has come apart from your Turbohaler.
Cleaning your Turbohaler
Wipe the outside of the mouthpiece once a week with a dry tissue. Do not use water or liquids.
When to start using a new Turbohaler
• When a red mark first appears in the indicator window under the mouthpiece, there are about 20 doses left. You will need to see your doctor for another prescription.
• When the red mark reaches the bottom of the indicator window, your Turbohaler is empty and you must start using your new Turbohaler.
S0566-100-IT-PIL-05.08.2015

• The grip will still twist and ‘click' even when your Turbohaler is empty.
• The sound that you hear as you shake your Turbohaler is produced by a drying agent and not the medicine. Therefore the sound does not tell you how much medicine is left in your Turbohaler.
• If you load your Turbohaler more than once by mistake before taking your dose, you will still only receive one dose.
If you use more Bricanyl Turbohaler than you should
If you use more Bricanyl Turbohaler than you should, contact your doctor or pharmacist for advice.
If you forget to use Bricanyl Turbohaler
• If you forget to take a dose, take it as soon as you remember. However, if it is nearly time for your next dose, skip the missed dose.
• Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Important side effects to look out for:
• Allergic reactions. The signs may include a swollen face, skin rash, breathing problems, low blood pressure (feeling faint) and collapse. It is not known exactly how often this happens. If this happens to you, stop using Bricanyl Turbohaler and see a doctor straight away.
• Sudden wheezing soon after inhaling your dose of Bricanyl Turbohaler.
It is not known exactly how often this happens. If this happens to you, stop using Bricanyl Turbohaler and see a doctor straight away.
Other possible side effects:
Very Common (may affect more than 1 in 10 people)
• Trembling or shaking.
• Headache.
Common (may affect up to 1 in 10 people)
• Pounding or rapid heart beat (palpitations).
• Cramp or feeling tense.
• Low levels of potassium in your blood which may cause muscle weakness, thirst, or ‘pins and needles'.
Not known (frequency cannot be estimated from the available data)
• Unusual or irregular heart beats.
• Chest pain (due to heart problems such as angina). Tell your doctor if you develop this symptom whilst receiving treatment with Bricanyl Turbohaler, but do not stop using this medicine unless told to.
• Feeling sick (nausea).
• Mouth and throat irritation.
• Changes in sleeping patterns and changes in behaviour, such as feeling agitated, restless or hyperactive.
Do not be concerned by this list of side effects. You may not get any of them.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Bricanyl Turbohaler
• Keep out of the sight and reach of children.
• Do not store above 30°C.
• Replace the cover properly after use.
• Do not use Bricanyl Turbohaler after the expiry date printed on the packaging. The expiry date refers to the last day of that month.
• If the turbohaler does not work properly or shows signs of deterioration, you should seek the advice of your pharmacist who will advise what to do.
• Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer required. This will help to protect the environment.
6. Contents of the pack and other information What Bricanyl Turbohaler contains
The active substance is terbutaline sulphate. Each inhalation contains 0.5mg of terbutaline sulphate. Bricanyl Turbohaler does not contain any other ingredients.
What Bricanyl Turbohaler looks like and the contents of the pack
Bricanyl Turbohaler is a multi-dose, dry powder inhaler with a white body and blue turning grip. The bottom of the turning grip has the number 1 embossed in Braille. Each Turbohaler contains 100 inhalations.
Manufacturer:
AstraZeneca AB, Sodertalje, Sweden or
AstraZeneca Dunkerque Production - Dunkerque, France.
Procured from within the EU and repackaged by: Amimed Direct Ltd, Hendon, London, NW9 6AQ.
Product Licence holder: Sam Pharma Ltd, Unit 20 Garrick Industrial Estate, Irving Way, Hendon, London, NW9 6aQ.
POM PL No: 33902/0566
This leaflet was last revised in: 05/08/2015
Bricanyl® and Turbohaler® are both registered trademarks of the AstraZeneca group of companies.
S0566-100-IT-PIL-05.08.2015