Brimonidine Tartrate 0.2% W/V Eye Drops
Please read this leaflet carefully before you start using this medicine.
Keep this leaflet. You may need to read it again. If you have further questions, please ask your doctor or pharmacist. This medicine has been prescribed for you personally and you should not pass it on to others. It may harm them, even if their symptoms are the same as yours. If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist. The medicine will be called Brimonidine in this leaflet.
In this leaflet:
1. What Brimonidine is and what it is used for
2. Before you use Brimonidine
3. Howto use Brimonidine
4. Possible side effects
5. Howto store Brimonidine
6. Further information
1. WHAT BRIMONIDINE IS AND WHAT IT IS USED FOR
Brimonidine Eye Drops are a sterile, preserved, aqueous solution used as eye drops. The active ingredient brimonidine tartrate works by reducing pressure within the eyeball. It is used to reduce pressure in the eye in the conditions of glaucoma or ocular hypertension. The medicine may be used alone or in conjunction with another eye drop that reduces pressure in the eye.
2. BEFORE YOU USE BRIMONIDINE Do not use
• if you are allergic to Brimonidine orto any of the other ingredients (see section 6. for more details)
• if you are taking a monoamine oxidase inhibitor medicine (MAOI) or a tricyclic antidepressant medicine (you should check with your doctor if you are taking any medicines for depression)
• in new bom babies or children under2years Take special care with Brimonidine
Before you use Brimonidine please tell your doctor If: e you suffer from depression e you suffer from heart disease or heart problems
e you suffer from dizziness or light-headedness on standing up (due to a fall in blood pressure) e yousufferfrom reduced blood supplyto the brain
e you suffer from blood vessel disease in the limbs, or from poor blood circulation which makes the fingers or toes numb and pale
• you are pregnant or breast-feeding
• you have liver or kidney problems.
• it is intended for use in a child between the ages of 2 and 12, because it is not usually recommended for use in this age group
Taking or using other medicines:
Check with your doctor before using the eye drops if you are taking or using any other medicines, in particular
• a monoamine oxidase inhibitor (MAOI) medicine or any other antidepressant
• sedative, barbitu rate or hypnotic medicines to help you sleep or to sedate you
• chlorpromazine, methylphenidate or reserpine (for mental/personality disorders)
• painkillers
• medicines for treating a heart condition or for lowering blood pressure
• medicines which work in the same way as brimonidine, eg.prazosin and isoprenaline
• If you are to be given anaesthetics, make sure you tell the doctor you are using these eye drops.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without prescription.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking any medicines.
Brimonidine should not be used during pregnancy and breast-feeding unless considered essential by your doctor.
Using Brimonidine with food and drink
The effects of alcohol may be increased if you drink whilst using Brimonidine.
Driving and using machines
Do not drive or operate machines if you feel drowsy or have blurred vision after using Brimonidine. These effects may seem worse at night or in reduced lighting.
Important information about some of the ingredients of Brimonidine Eye Drops The eye drops contain benzalkonium chloride as preservative which may cause eye irritation. Avoid contact with soft contact lenses. Remove contact lenses before using the eye drops and wait at least 15 minutes before reinserting. The preservative is known to discolour soft contact lenses.
3. HOW TO USE BRIMONIDINE
Always use Brimonidine exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Adult dose: Put one drop into each affected eye twice daily, approximately 12 hours apart. If you are using in combination with another eye drop medicine, wait 5-15 minutes before applying the second eyedrop.
Children: Brimonidine must not be used in infants under 2 years of age. It is not recommended for use in children between 2 and 12 years of age.
Instructions for use (Please also refer to pictograms at the end of the leaflet)
• First wash your hands
• Avoid touching the eye (or any other surface) with the tip of the bottle
• If you wear soft contact lenses, they should be removed before using the eye drops and wait at least 15 minutes before reinserting
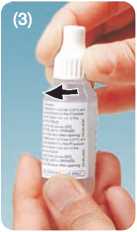
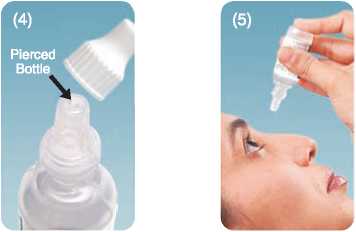
• These drops are supplied as a sealed bottle with a spiked cap. When using the bottle for the first time, screw the cap down tightly in order to pierce the tip of the bottle
• Tiltyourhead back and lookatthe ceiling
• Pull the lower eyelid gently downwards
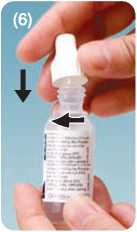
• Hold the bottle upside down above the eye and gently squeeze the bottle to release a drop into your eye
• Keep the affected eye closed and press your fingertip against the inside corner of the closed eye, and hold for 1 minute
• Repeatfortheothereyeifnecessary
• Replace and tighten the cap immediately after use.
Be careful not to touch the tip of the bottle on your eye or on any other surface.
Ocular solutions if handled wrongly, can become contaminated by common bacteria and cause eye infections. If you do develop any other eye condition whilst using this product, see your doctor immediately.
If you use more Brimonidine than you should : In adults an overdose due to the

use of the eye drops is unlikely. In children overdose has been reported in those receiving brimonidine as part of treatment for glaucoma. Contact your doctor immediately if a child develops signs of sleepiness, floppiness, low temperature or breathing difficulties. If you use too many drops or if the eye drops are accidentally swallowed, you should contact your doctor.
If you forget to use Brimonidine Apply the drops as soon as you remember. However, if it is almost time for your next dose, do not double your dose and carry on with the normal schedule dose.
4. POSSIBLE SIDE EFFECTS
Like all medicines Brimonidine can cause side effects, although not everybody gets them. If you experience a rare (these may affect between 1 in 1,000 and 1 in 10,000 patients) but serious allergic reaction (difficulty breathing, closing of the throat, swelling of the lips, tongue, or face or hives) to brimonidine, stop using the medication and contact your doctor immediately.
Please tell your doctor if you notice any of the following side effects:
Very common: (these may affect more than 1 in 10 patients)
• an allergic reaction in the eye causing eye redness, burning, stinging, follicles or white spots on the membrane covering the inside of the eyelid/white of the eye, blurred vision, a feeling of something in the eye or itching
• headache, tiredness, drowsiness and dry mouth.
Common: (these may affect between 1 in 10 and 1 in 100 patients)
• changes to the surface of the eye, swollen or red eyelid, abnormal vision, sticky, weepy or watery eyes, sensitivity to light, irritation, pain, whitening of the membrane covering the inside of the eyelid/white of the eye
• dizziness, feeling or being sick, general weakness
• cold-like symptoms orabnormal taste.
Uncommon: (these may affect between 1 in 100 and 1 in 1,000 patients)
• depression
• palpitations or changes in heart rate
• dry nose or general allergic reactions
Rare: (may affect between 1 in 1,000 and 1 in 10,000 patients)
• shortness of breath
Very rare: (these may affect less than 1 in 10,000 patients) e eye inflammation or a reduction in pupil size, e fainting, high or low blood pressure or sleeplessness.
Some of the effects on the eye may be due to an allergy to the active ingredient or to any of the other ingredients.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
5. HOW TO STORE BRIMONIDINE
Do not store above 25° C.
Discard within 28 days of opening.
Keep all medicines out of the reach and sight of children.
The product should not be used after the expiry date. (This is printed on both the bottle label and on the carton the bottle is packed in).
6. FURTHER INFORMATION What Brimonidine contains
Active ingredient is brimonidine tartrate 0.2% (2 mg/ml). Also contains 0.005% benzalkonium chloride (as preservative), polyvinyl alcohol, sodium citrate, citric acid, sodium chloride, sodium hydroxide and water for injection.
What Brimonidine looks like and contents of the pack
Each bottle contains 5 ml of the clear, greenish yellow coloured, eye drop solution.
Marketing Authorisation Holder and Manufacturer
FDC International Ltd, Unit 6, Fulcrum 1, Solent Way, Whiteley, Fareham, Hampshire,
P015 7FE
PL number: 15872/0018
Hard to see or read the leaflet? Call + 44(0) 1489 565222 for help.
This leaflet was last approved in : 03/2014
MODE OF USE
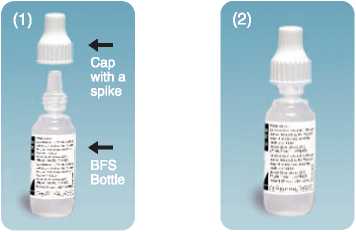
Bottle as received
Tighten the cap on the nozzle till the cap touches the shoulder.
Colour
CMYK
Black
The spike in the cap Tilt head backwards. Dispense drops Replace cap after will pierce the tip with gentle pressure. Do not touch every use, and screw of the bottle. dropper tip to the surface of the eye. the cap down