Brinzolamide 10 Mg/Ml Eye Drops Suspension
140 mm >
X
X
n
12 mm
PACKAGE LEAFLET: INFORMATION FOR THE USER Brinzolamide 10 mg/ml Eye drops suspension
Brinzolamide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Brinzolamide 10 mg/ml Eye drops suspension is and what it is used for
2. What you need to know before you use Brinzolamide 10 mg/ml Eye drops suspension
3. How to use Brinzolamide 10 mg/ml Eye drops suspension
4. Possible side effects
5. How to store Brinzolamide 10 mg/ml Eye drops suspension
6. Contents of the pack and other information
1. What Brinzolamide 10 mg/ml Eye drops suspension is and what it is used for
Brinzolamide 10 mg/ml Eye drops suspension contains brinzolamide which belongs to a group of medicines called carbonic anhydrase inhibitors. It reduces pressure within the eye.
t_^.Brinzolamide 10 mg/ml Eye drops suspension is used to treat high pressure in the _
12 mm eye. This pressure can lead to an illness called glaucoma. 12 mm
If the pressure in the eye is too high, it can damage your sight.
2. What you need to know before you use Brinzolamide 10 mg/ml Eye drops suspension
Do not use Brinzolamide 10 mg/ml Eye drops suspension.
- if you have severe kidney problems.
- if you are allergic to brinzolamide or any of the other ingredients of this medicine (listed in section 6).
- if you are allergic to medicines called sulphonamides. EXAMPLES include medicines used to treat diabetes and infections and also diuretics (water tablets). Brinzolamide 10 mg/ml Eye drops suspension may cause the same allergy.
- if you have too much acidity in your blood (a condition called hyperchloraemic acidosis) If you have further questions ask your doctor for advice.
Warnings and Precautions
Talk to your doctor or pharmacist before using Brinzolamide 10 mg/ml Eye drops suspension:
- if you have kidney or liver problems.
- if you have dry eyes or cornea problems.
- if you are taking other sulphonamide medicines.
Children and adolescents
Brinzolamide 10 mg/ml Eye drops suspension is not to be used by infants, children or adolescents under 18 of years of age unless advised by your doctor.
Other medicines and Brinzolamide 10 mg/ml Eye drops suspension
Tell your doctor or pharmacist if you are taking or have recently taken or might take any other medicines including medicines obtained without a prescription.
If you are taking another carbonic anhydrase inhibitor (acetazolamide or dorzolamide, see section 1: What Brinzolamide 10 mg/ml Eye drops suspension is and what it is used for), talk to your doctor.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
LULU 7.

460 mm
Women who may become pregnant are advised to use effective contraception during treatment with Brinzolamide 10 mg/ml Eye drops suspension. The use of Brinzolamide 10 mg/ml Eye drops suspension is not recommended during pregnancy or breast-feeding. Do not use Brinzolamide 10 mg/ml Eye drops suspension unless clearly indicated by your doctor.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Do not drive or use machines until your vision is clear. You may find that your vision is blurred for a time just after using Brinzolamide 10 mg/ml Eye drops suspension.
Brinzolamide 10 mg/ml Eye drops suspension may impair the ability to perform tasks requiring mental alertness and/or physical coordination. If affected, take care when driving or using machines.

Brinzolamide 10 mg/ml Eye drops suspension contains benzalkonium chloride
Brinzolamide 10 mg/ml Eye drops suspension contains a preservative (benzalkonium chloride) which may cause eye irritation and is known to discolour soft contact lenses. Contact with soft contact lenses should be avoided. If you wear contact lenses you should remove them prior to the application of Brinzolamide 10 mg/ml Eye drops suspension and wait at least 15 minutes after instillation of the dose before putting your lenses back in.
3. How to use Brinzolamide 10 mg/ml Eye drops suspension
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist or nurse if you are not sure.
Only use Brinzolamide 10 mg/ml Eye drops suspension for your eyes. Do not swallow or
The recommended dose is
1 drop in the affected eye or eyes twice a day - morning and night.
Use this much unless your doctor told you to do something different. Only use Brinzolamide 10 mg/ml Eye drops suspension in both eyes if your doctor told you to. Use it for as long as your doctor told you to.
How to use
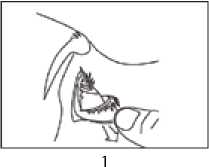
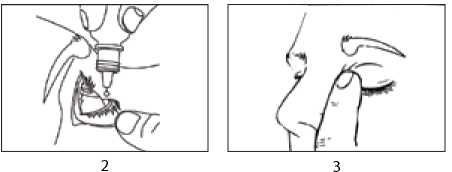
1. Get the eye drops bottle. You must not use the bottle if the tamper-proof seal on the bottle neck is broken before you use it the first time.
2. Wash your hands.
3. Shake well the bottle and twist off the cap. After the cap is removed, if the tamper evident snap collar is loose, remove before using product.
4. Bend your head backwards and gently pull your lower eyelid down until there is a small "pocket" (picture 1). It is easier if you sit or stand in front of a mirror.
5. Hold the bottle upside down between your thumb and forefinger, above one eye. Squeeze one drop into the formed pocket. Do not touch your eye or eyelashes, surrounding areas or anything else with the dropper tip (picture 2).
6. If a drop misses your eye, try again.
7. Let go of the eyelid and gently press a finger to the corner of your eye, by the nose. This helps to stop Brinzolamide getting into the rest of the body (picture 3).
8. If you take drops in both eyes, repeat the steps 4 to 7 for your other eye.
9. Tighten the cap on the bottle immediately after use.
10. Use up one bottle before opening the next bottle.
If you are using other eye drops, leave at least 5 minutes between putting in Brinzolamide 10 mg/ml Eye drops suspension and the other drops. Eye ointments should be administered last.
A
12 mm
<
A
140 mm — 12 mm
>
T
If you use more Brinzolamide 10 mg/ml Eye drops suspension than you should
If you get too much in your eyes, rinse it all out with warm water. Do not put in any more drops until it is time for your next regular dose.
If you forget to use Brinzolamide 10 mg/ml Eye drops suspension
use a single drop as soon as you remember, and then go back to your regular routine. Do not use a double dose to make up for a forgotten dose.
If you stop using Brinzolamide 10 mg/ml Eye drops suspension
If you stop using Brinzolamide 10 mg/ml Eye drops suspension without speaking to your doctor, the pressure in your eye will not be controlled which could lead to loss of sight.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everyone gets them. The following side effects have been seen with Brinzolamide 10 mg/ml Eye drops suspension:
Common side effects
(may affect up to 1 in 10 people)
Effects in the eye: blurred vision, eye irritation, eye pain, eye discharge, itchy eye, dry eye, abnormal eye sensation, redness of the eye General side effects: bad taste
Uncommon side effects
(may affect up to 1 in 100 people)
Effects in the eye: sensitivity to light, inflammation or infection of the conjunctiva, eye swelling, eyelid itching, redness or swelling, growth on surface of eye, increased pigmentation of the eye, tired eyes, eyelid crusting, increased tear production.
General side effects: decreased or reduced heart function, palpitations, decreased heart rate, difficulty breathing, shortness of breath, cough, decreased red blood cell count in blood, increased chlorine level in blood, dizziness, drowsiness, difficulty with memory, depression, nervousness, generalized weakness, fatigue, feeling abnormal, pain, shaking, decreased sex drive, male sexual difficulty, cold symptoms, chest congestion, sinus infection, throat irritation, throat pain, abnormal or decreased sensation in mouth, inflammation of the lining of the oesophagus, abdominal pain, nausea, vomiting, upset stomach, frequent bowel movements, diarrhoea, intestinal gas, digestive disorder, kidney pain, muscle pain, muscle spasms, back pain, nose bleeds, runny nose, stuffy nose, sneezing, rash, abnormal skin sensation, itching, headache, dry mouth.
Rare side effects
(may affect up to 1 in 1,000 people)
Effects in the eye: corneal swelling, double or reduced vision, abnormal vision, decreased eye sensation, swelling around the eye, increased pressure in eye, damage to the optic nerve
General side effects: memory impairment, drowsiness, chest pain, upper respiratory tract congestion, sinus congestion, nasal congestion, dry nose, ringing in ears, hair loss, generalized itching, feeling jittery, irritability, irregular heart rate, body weakness, difficulty sleeping.
Not known
(frequency cannot be estimated from the available data):
Effects in the eye: eyelid abnormality, visual disturbance, corneal disorder, eye allergy, decreased growth or number of eyelashes
General side effects: increased allergic symptoms, decreased sensation, tremor, loss or decrease in taste, decreased blood pressure, increased blood pressure, increased heart rate, joint pain, asthma, pain in extremity, skin redness, inflammation, or itching, abnormal liver blood tests, swelling of the extremities, frequent urination, decreased appetite.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting systems listed below:
United Kingdom:
Yellow Card Scheme
<-►
12 mm
Website: www.mhra.gov.uk/yellowcard
12 mm
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Brinzolamide 10 mg/ml Eye drops suspension
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the bottle and box after "EXP". The expiry date refers to the last day of the month.
This medicine does not require any special storage conditions.
You must throw away a bottle four weeks after you first opened it, to prevent infections. Write down the date you opened each bottle in the space below and on the bottle label and box. For a pack containing a single bottle, write only one date.
Opened (1) Opened (2) Opened (3)
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Brinzolamide 10 mg/ml Eye drops suspension contains The active substance is brinzolamide. Each millilitre contains 10 mg of brinzolamide. The other ingredients are: benzalkonium chloride, carbomer 974P, edetate disodium, Mannitol (E421), purified water, sodium chloride. Tiny amounts of hydrochloric acid or sodium hydroxide are added to keep acidity levels (pH levels) normal.
What Brinzolamide 10 mg/ml Eye drops suspension looks like and the contents of the pack
Brinzolamide 10 mg/ml Eye drops suspension is a milky liquid (a suspension) supplied in a pack containing a 5 ml plastic bottle with a screw cap, or in a pack containing three 5 ml plastic bottles with screw caps. Not all pack sizes may be marketed.
The Marketing Authorization Holder and Manufacturer
Marketing Authorisation Holder: Wockhardt UK Ltd, Ash Road North, Wrexham,
LL13 9UF, UK.
Manufacturer
Lusomedicamenta Sociedade Tecnica Farmaceutica, S.A., Rua Norberto de Oliveira, no 1/5, Povoa de Santo Adriao, 2620-111, Portugal
Other sources of information:
To listen to or request a copy of this leaflet in Braille, large print or audio please call, free of charge: 0800 198 5000 (UK Only)
Please be ready to give the following information:
|
Product name |
Reference number |
|
Brinzolamide 10 mg/ml Eye Drops Suspension |
29831/0572 |
This is a service provided by the Royal National Institute of Blind People.
This leaflet was last revised in 05/2015.
i
107028/1
A
12 mm