Buccastem M Buccal Tablets
Package leaflet: Information for the user □ ALLIANCE
Buccastem® M Buccal Tablets
(PROCHLORPERAZINE MALEATE 3MG, EQUIVALENT TO 1.85 MG PROCHLORPERAZINE BASE)
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Always take this medicine exactly as described in this leaflet or as your doctor or pharmacist has
I told you.
- Keep this leaflet. You may need to read it again.
- Ask your pharmacist if you need more information or advice.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Buccastem M Tablets are and what they are used for
2. What you need to know before you take Buccastem M Tablets
3. How to take Buccastem M Tablets
4. Possible side effects
5. How to store Buccastem M Tablets
6. Contents of the pack and other information
| 1. What Buccastem M Tablets are and what they are used for
Buccastem M Tablets are effective in treating nausea (feeling sick) and vomiting (being sick) associated with migraine.
Buccastem M Tablets contain prochlorperazine maleate. Prochlorperazine belongs to a large group of drugs known as phenothiazines, which have a variety of effects.
| 2. What you need to know before you take Buccastem M Tablets
You should only use this medicine if migraine has previously been diagnosed by your doctor.
Do not take Buccastem M Tablets:
• if you are allergic to prochlorperazine maleate or any of the other ingredients of this medicine (listed in section 6)
• if you are pregnant, or you think you might be pregnant, or breast feeding
• if you have problems with your liver
• if you have blood problems
• if you suffer from epilepsy, Parkinson’s Disease or glaucoma
• if you have problems with your prostate gland
• if you are under 18 years of age.
Warnings and precautions
Talk to your doctor or pharmacist before taking Buccastem M Tablets:
• if you are elderly
• if you have risk factors for a blood clot or stroke such as high blood pressure, high cholesterol levels, diabetes or smoking
• if you or someone else in your family has a history of blood clots, as medicines like these have been associated with formation of blood clots
• if you have a condition called myasthenia gravis, which causes muscle weakness
• if you have AIDS.
Buccastem M Tablets may cause you to be more sensitive to sunlight. When taking Buccastem M Tablets it is particularly important that you should avoid exposure to direct sunlight and use sunscreen.
Other medicines and Buccastem M Tablets
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
In particular, tell your doctor or pharmacist if you are taking:
• sedatives or tranquilisers, used to help you sleep (such as diazepam and temazepam)
• antihypertensive medicines, used to treat high blood pressure (such as atenolol, propranolol or clonidine)
• anticholinergic medicines, used for conditions such as depression (such as amitriptyline and imipramine)
• anticonvulsant medicines, used to prevent or treat fits (such as carbamazepine and lamotrigine)
• lithium (used to treat mania and depression)
• desferrioxamine (used to treat high levels of iron in your body)
• oral anticoagulants, to help prevent blood clots (such as warfarin)
• levodopa (used to treat Parkinson’s disease).
Buccastem M Tablets with food and drink
Buccastem M Tablets are best taken after food.
Do not drink alcohol when taking the tablets as it may interact with medicines like Buccastem M.
Pregnancy and breast feeding
If you are pregnant or thinking of becoming pregnant, you should not take Buccastem M Tablets.
Experience with Buccastem M Tablets is limited. You should therefore not take the tablets if you are breast-feeding.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Buccastem M Tablets can make you feel drowsy. Therefore you should avoid driving or using dangerous machines until you know how the tablets affect you.
Buccastem M Tablets contain sucrose
If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product.
| 3. How to take Buccastem M Tablets
Always take this medicine exactly as described in this leaflet or as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is one or two tablets twice a day for a maximum of 2 days, for adults aged 18 years or over.
Buccastem M Tablets are not recommended for children under 18 years of age.

INSTRUCTIONS FOR USE Please read carefully before taking the tablet(s).
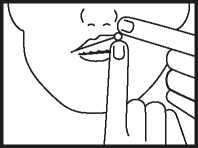
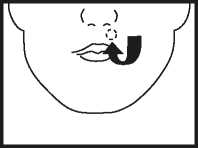
• Place the tablet high up along your top gum, under the upper lip either side of your mouth as indicated above. The tablet must not be swallowed whole or chewed.
• The tablet will soften and adhere to the gum. Allow it to dissolve slowly and completely - this may take between 1 and 2 hours. Most people find that after a few minutes they no longer notice the tablet.
• The tablet should not be moved about the mouth with the tongue as this will cause it to dissolve too quickly.
• If you wear dentures, the tablet may be placed in any comfortable position between your lip and gum.
The tablet(s) is best taken after meals.
If you take more Buccastem M Tablets than you should
If you accidentally take too many tablets you must seek medical attention immediately. Show any left-over medicines or the empty packet to the doctor.
If you forget to take Buccastem M Tablets
If you forget to take a dose do not double the dose next time. Just carry on taking the medicine as the doctor, pharmacist or nurse has told you.
| 4. Possible side effects |
Like all medicines, Buccastem M Tablets can cause side effects, although not everybody gets them.
Serious side effects
If you experience any of the following side effects, you should stop taking Buccastem M Tablets and contact your doctor immediately:
• Symptoms of an allergic reaction such as rash and swelling of the face, tongue or throat
• A high temperature, pale complexion, muscle stiffness and changes in levels of alertness, you may have developed a serious condition called neuroleptic malignant syndrome.
• Convulsions (fits).
• Signs of infection such as shivering, headache, sweating, high temperature, flushing, a sore throat or mouth and swollen glands.
• Blood clots in the veins especially in the legs (symptoms include swelling, pain and redness in the leg), which may travel through blood vessels to the lungs causing chest pain and difficulty in breathing.
• Symptoms of decreased sodium concentration in the blood, such as nausea and vomiting, headache, confusion, loss of energy, restlessness, muscle weakness, spasms or cramps, seizures and coma.
In elderly people with dementia, a small increase in the number of deaths has been reported for patients taking medicines of the same type as Buccastem M Tablets compared with those not taking these medicines.
Other side effects include:
• drowsiness
• dizziness
• dry mouth
• inability to sleep (insomnia)
• agitation
• skin reactions
• increased sensitivity to sunlight
• low blood pressure (this makes you feel dizzy or faint, particularly when you stand up), particularly in elderly or volume depleted patients (those who have lost both water and salts from the body)
• high blood sugar level, which can cause increased thirst, a dry mouth, needing to urinate frequently, tiredness and recurrent infections such as thrush
• a condition known as cholestasis where bile cannot flow properly in the body, and can cause itchiness, jaundice (yellowing of the skin and/or the whites of the eyes), pale stools and dark urine
• abnormal movements, tremors and muscle rigidity, and unusual movements of the face and tongue
• impotence, ejaculation problems and persistent/ painful erections
• local irritation to the gum and mouth.
Rare
• jaundice
• blood problems.
Very rare
• breast swelling (in men as well as in women)
• milk secretion from the breasts which is not due to breast-feeding
• absence of menstrual periods
• a feeling of restlessness or anxiety that can range from mild to severe.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
| 5. How to store Buccastem M Tablets |
Keep this medicine out of the sight and reach of children.
Keep the blisters in the outer carton in order to protect from light.
This medicinal product does not require any special temperature storage condition.
Do not use after the expiry date (EXP month/year) shown on the outer carton and blister.
The expiry date refers to the last day of that month.
| 6. Contents of the pack and other information |
What Buccastem M Tablets contain
Each tablet contains 3 mg of the active ingredient prochlorperazine maleate.
The other ingredients are: compressible sugar (sucrose), povidone K30, xanthan gum, locust bean gum, talc, magnesium stearate and riboflavin sodium phosphate.
What Buccastem M Tablets look like and contents of the pack
The buccal tablet is pale yellow and has JI on one side and is plain on the other.
This product is available from pharmacists in packs of 8 tablets.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder: Alliance Pharmaceuticals Limited, Avonbridge House, Bath Road, Chippenham, Wiltshire, SN15 2BB, UK.
Manufacturer: Dales Pharmaceuticals Limited, Snaygill Industrial Estate, Keighley Road, Skipton, BD23 2RW, United Kingdom.
This leaflet was last revised in January 2016. XXXXX
Buccastem and Buccastem M are registered trademarks of Alliance Pharmaceuticals Limited. Alliance and associated devices are registered trademarks of Alliance Pharmaceuticals Limited. © Alliance Pharmaceuticals Limited 2016