Budesonide 1 Mg Nebuliser Suspension
PACKAGE LEAFLET: INFORMATION FOR THE USER
Budesonide 0.5mg and 1mg Nebuliser Suspension
Read all of this leaflet carefully before you start using this medicine because It contains Important Information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
• The antibiotic medicines, erythromycin and clarithromycin
• Other medicines which help you to breathe
• Oestrogens and contraceptive steroids
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. If you become pregnant whilst taking this medicine you should tell your doctor as soon as possible.
Driving and using machines
Inhaled budesonide has no or negligible influence on the ability to drive and use machines.
3. How to use Budesonide Nebuliser Suspension
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Your doctor will advise you of the correct dose which will depend on how bad your asthma is.
Asthma
You may find that your asthma improves within 3 days but it can take between 2-4 weeks before the full effect is achieved. It is important that you keep taking your medicine as instructed by your doctor even if you feel better.
Adults (including the elderly) and adolescents of 12 years and over:
The usual dose is 0.5-2.0mg of budesonide daily. This dose will normally be taken on two separate occasions during the day although if your asthma is stable and not severe your doctor may advise you to take this medicine once a day. Your doctor will tell you how and when it is best to take your medicine and you should always follow their instructions.
Infants and children (aged 6 months to 11 years):
The usual dose is 0.25-1.0mg of budesonide daily. The doctor will advise you on how your child should take their medicine but this will usually be on two separate occasions during the day. However if their asthma is stable and not severe your doctor may advise that they should take this medicine once a day.
Pseudocroup:
The usual dose for infants and children with pseudocroup is 2 mg. This is given as a single dose or as two 1 mg doses separated by 30 minutes.
The treatment can be repeated every 12 hours as necessary until improvement is seen, for a maximum of 36 hours.
Instructions for use:
Your medicine must be used with a jet nebuliser. The "mist” produced is then inhaled through a mouthpiece or face mask. Ultrasound nebulisers should not be used with this medicine.
To take your medicine follow these steps:
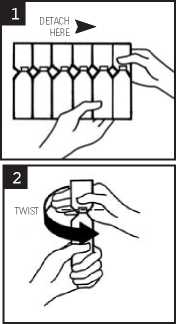
1. Break off a single ampoule from the strip, leaving the rest in the foil sachet (see diagram 1)
2. Shake the ampoule gently
3. Open the ampoule by twisting off the top (see diagram 2)
4. Squeeze all of the liquid from the ampoule into the nebuliser cup. Replace the top of the nebuliser cup and dispose of the empty ampoule
What is in this leaflet:
1. What Budesonide Nebuliser Suspension is and what it is used for
2. What you need to know before you use Budesonide Nebuliser Suspension
3. How to use Budesonide Nebuliser Suspension
4. Possible side effects
5. How to store Budesonide Nebuliser Suspension
6. Contents of the pack and other information
1. What Budesonide Nebuliser Suspension is and what it is used for
|
Budesonide 0,5mg and 1mg ampoules PIL - UK |
colours/plates: 1.black | ||
|
^actavis trusting value tnp/wmMouliats |
item no: AAAI1478 |
dimensions: 120x315 |
2. |
|
print proof no: 2 |
pharmacode: |
3. | |
|
4. | |||
|
origination date: 20.07.15 |
min pt size: 8pt |
5. | |
|
originated by: dje |
6. | ||
|
approved for print/date |
revision date: 25.11.15 |
Technical Approval |
Non Printing Colours |
|
1. | |||
|
revised by: dje |
date sent: 20.07.15 | ||
|
2. | |||
|
supplier: Catalent |
3. | ||
* Please note the technical approval is provided by the supplier and is valid on the date indicated.
Any technical changes madeby the supplier after approval are not the responsibility of the Artwork Studio.
5. How to store Budesonide Nebuliser Suspension
5. Connect one end of the cup to the mouthpiece or face mask and the other end to the air pump
6. Gently shake the cup once more then turn on the nebuliser. Breathe in the "mist” calmly and deeply using the mouthpiece or face mask
7. When no more mist comes out of the mouthpiece or face mask, your treatment is complete
8. Rinse your mouth with water (spit out the water - do not swallow it) and brush your teeth. If you have used a face mask, you should also wash your face well. It is important to do this as it can reduce the risk of some side effects associated with this medicine
9. You should clean the nebuliser after each use. Wash the nebuliser container and mouthpiece or face mask in warm water using a mild detergent in accordance with the manufacturer's instructions. The nebuliser should then be rinsed well and dried by connecting the nebuliser container to the air pump.
It is important that you always follow the manufacturer's instructions that come with the nebuliser. If you are not sure about how to use the nebuliser, talk to your doctor or pharmacist.
Your doctor may also prescribe the following:
• Your doctor may consider adding steroid tablets to your treatment during periods of stress (e.g. if you have an infection), or if you have been taking a high dose of an inhaled steroid for a long time, or before an operation.
• If you have been taking steroid tablets for your asthma, your doctor may reduce the number of tablets that you take once you start to use Budesonide Nebuliser Suspension. You might experience some symptoms as a result of this including a stuffy or runny nose, a lack of energy, depression, eczema (a type of skin rash) and joint and/or muscle pain. If any of these symptoms bother you or persist, please contact your doctor.
• Your doctor may ask you to mix this medicine with solutions containing other active substances which work on the respiratory system such as salbutamol, terbutaline, sodium cromoglicate and ipratropium bromide. If so follow their instructions carefully.
You must not mix this medicine unless specifically instructed by your doctor.
If you use more Budesonide Nebuliser Suspension than you should
Contact your doctor or pharmacist as soon as possible. Remember to take the pack and any remaining ampoules with you. It is important that you take your dose as stated on the pharmacist's label or as advised by your doctor. You should not increase or decrease your dose without seeking medical advice.
If you forget to use your Budesonide Nebuliser Suspension
Do not take a double dose to make up for a forgotten dose. Simply take your next dose on time.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
I 4. Possible side effects I
Like all medicines, this medicine can cause side effects, although not everybody gets them.
All medicines can cause allergic reactions, although serious allergic reactions are very rare. Tell your doctor immediately if you get any sudden wheeziness, difficulty in breathing, swelling of the eyelids, face or lips, rash or itching (especially affecting your whole body).
Rarely, inhaled drugs such as budesonide can cause acute wheezing and/or shortness of breath. If this occurs, stop using this medicine immediately and seek medical advice.
The following side effects have been reported.
Common: may affect up to 1 in 10 people Soreness and/or irritation in the mouth (including oral thrush), hoarseness, throat irritation, difficulty in swallowing and cough.
Rare: may affect up to 1 in 1,000 people Skin reactions including itching, rash, bruising, inflammation, redness of the skin and/or skin eruptions, swelling, slowing of growth in children and adolescents, hypersensitivity (an allergy to the medicine) and bronchospasm (tightening of the muscles in the airways resulting in wheezing), voice problems.
Suppression of your adrenal gland (a small gland next to the kidney) can also occur. The major symptoms of adrenal suppression include headaches, tiredness, feeling and being sick, weight loss, stomach pain and lack of appetite.
Feeling restless, nervous, over-excited, irritable or depressed (these effects are more likely to occur in children)
Very rare: may affect up to 1 in 10,000 people Decrease in bone mineral density (thinning of the bones).
Not known: frequency cannot be estimated from the available data
Cataracts (clouding of the lens in the eye), glaucoma (increased pressure in the eye), aggression, increased motor activity (difficulty to stay still), sleeping problems or feeling anxious.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www. mhra.gov.uk/vellowcard
By reporting side effects you can help provide more information on the safety of this medicine.
Keep this medicine out of the sight and reach of children.
• Do not use this medicine after the expiry date which is stated on the carton and foil sachet after Exp. The expiry date refers to the last day of that month
• Store the ampoules in their foil sachet and their original carton in order to protect from light and moisture
• Once you have opened a single ampoule it should be used within 12 hours. After this time, the ampoule and any remaining contents should be thrown away
• Once a foil sachet has been opened, the ampoules inside should be used within 3 months (it is a good idea to mark the opening date on the foil sachet to help you remember).
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
AAAI1478
|
Budesonide 0,5mg and 1mg ampoules PIL - UK |
colours/plates: 1.black | ||
|
^actavis trusting value tnp/wmMouliats |
item no: AAAI1478 |
dimensions: 120x315 |
2. |
|
print proof no: 2 |
pharmacode: |
3. | |
|
4. | |||
|
origination date: 20.07.15 |
min pt size: 8pt |
5. | |
|
originated by: dje |
6. | ||
|
approved for print/date |
revision date: 25.11.15 |
Technical Approval |
Non Printing Colours |
|
1. | |||
|
revised by: dje |
date sent: 20.07.15 | ||
|
2. | |||
|
supplier: Catalent |
technically app. date3: |
3. | |
* Please note the technical approval is provided by the supplier and is valid on the date indicated.
Any technical changes madeby the supplier after approval are not the respons'ib'ilty of the Artwork Studo.
Budesonide belongs to a group of steroids called glucocorticoids which can be used to reduce or prevent inflammatory reactions (inflammation) in the lungs.
Your medicine is used for the treatment of asthma. It is used in patients where other types of inhaler, such as a pressurised inhaler or an inhaler containing a dry powder are unsatisfactory or inappropriate.
This medicine may also be used for the treatment in hospitals of very serious cases of pseudocroup (a disease of the throat that may cause difficulty in breathing).
2. What you need to know before you use Budesonide Nebuliser Suspension
Do not use Budesonide Nebuliser Suspension:
• If you are allergic to budesonide or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions:
Talk to your doctor or pharmacist before using Budesonide Nebuliser Suspension if any of the following apply to you:
• If you have or have had tuberculosis
• If you have or have had a liver disease or problems with your liver
What Budesonide Nebuliser Suspension contains
• The active substance is budesonide
The other ingredients are disodium edetate, sodium chloride, polysorbate 80, citric acid, sodium citrate and water for injection.
Budesonide 0.5mg Nebuliser Suspension contains 0.5mg of budesonide (0.25mg/ml) as the active substance in each 2ml ampoule.
Budesonide 1mg Nebuliser Suspension contains 1.0mg of budesonide (0.5mg/ml) as the active substance in each 2ml ampoule.
What Budesonide Nebuliser Suspension looks like and contents of the pack
Your medicine comes in the form of plastic ampoules containing 2ml of a white to off-white suspension to be nebulised (made into a fine mist for inhalation).
The ampoules are packed in strips of 4, 5, 8, 10 or 12 inside a foil sachet which is then packed into a carton. The cartons are available in pack sizes containing 5,
20, 24, 40 (2 x 20) or 60 ampoules. Not all pack sizes may be marketed.
Marketing Authorisation Holder:
Breath Limited, Whiddon Valley, Barnstaple, North Devon, EX32 8NS, United Kingdom.
Manufacturer:
Qualiti (Burnley) Limited, Talbot Street, Briercliffe, Burnley,
BB10 2JY, United Kingdom
This leaflet was last revised in November 2015.