Buprenorphine 2 Mg Sublingual Tablets
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
044BP1982C
Package leaflet: Information for the user
Buprenorphine 2 mg sublingual tablets
Buprenorphine 8 mg sublingual tablets
Buprenorphine
What is in this leaflet:
1. What Buprenorphine is and what it is used for
2. What you need to know before you take Buprenorphine
3. How to take Buprenorphine
4. Possible side effects
5. How to store Buprenorphine
6. Contents of the pack and other information
1. What Buprenorphine is and what it is used for
Buprenorphine belongs to a group of medicines called opioid analgesics (also known as ‘opiates' or ‘narcotics'). Opioid analgesics, such as morphine or diamorphine (heroin), are often subject to abuse, which can lead to dependence (addiction). If you are addicted to these drugs, you need a regular dose to feel ‘normal', otherwise you will develop withdrawal symptoms within a day or so of the last dose. Withdrawal symptoms include sweating, feeling hot and cold, runny eyes and nose, feeling or being sick, diarrhoea, stomach cramps, poor sleep and just feeling awful.
Buprenorphine is used as a substitution (replacement) treatment in patients who are addicted to opioid drugs such as heroin and morphine. The tablets prevent or reduce the unpleasant withdrawal symptoms experienced when addicts stop using opioid drugs.
Treatment with Buprenorphine may form one aspect of a specialist support programme aimed at resolving opioid addiction.
Treatment with Buprenorphine is intended for use in adults and adolescents aged 16 years or older who have agreed to be treated for addiction.
2. What you need to know before you take Buprenorphine
Do not take Buprenorphine
- if you are allergic to buprenorphine or any of the other ingredients of this medicine (listed in section 6). if you have severe breathing problems
- if you have severe liver disease
- if you are an alcoholic or regularly drink large amounts of alcohol (more than two drinks per day for men and more than one drink per day for women (e.g. one drink = one 350 ml bottle of beer (4.5% alcohol) or one 150 ml glass of wine (12.9% alcohol))
- if you have delirium tremens (confusion and shaking after stopping drinking alcohol and hallucinations (seeing and hearing things that are not there)).
Warnings and precautions
Tell your doctor or pharmacist before taking Buprenorphine if you have any of the following
illnesses before treatment or if you develop them during treatment, as your doctor may need to
reduce your dose of Buprenorphine or you may need extra treatment to control them:
- asthma or any other breathing problems
- any kidney problems
- any liver problems
- head injury or brain disease
- low blood pressure
- in men: urinary disorders (especially linked to enlarged prostate).
Misuse and abuse
Misuse, especially by injection and at high dose is dangerous and could be fatal.
- some people have died from respiratory failure (inability to breathe) because they misused buprenorphine or took it in combination with other central nervous system depressants such as alcohol, benzodiazepines (medicines used to treat anxiety or sleep disorders) or other opioids
- cases of severe liver injury have been reported following misuse, especially by intravenous route and at a high dose. These injuries may be worsened by viral infections (chronic hepatitis C), alcohol abuse, anorexia or some medicines (e.g. antiretroviral medicines, acetylsalicylic acid (aspirin), amiodarone, isoniazid, valproate). If you have symptoms of severe fatigue, no appetite, itching, or if your skin or eyes look yellow, tell your doctor immediately so that you can receive the proper treatment.
Buprenorphine can cause:
- withdrawal symptoms if you take it less than 4-6 hours after you use a narcotic such as morphine or heroin or less than 24 hours after you use methadone
- sleepiness which may be worse if you also drink alcohol or take tranquillisers or anti-anxiety medicines. If you are drowsy, do not drive or operate machinery.
- sudden drop in blood pressure, causing you to feel dizzy if you get up too quickly from sitting or lying down
- drug dependency
- a positive reaction to ‘anti-doping' tests (athletes should be aware).
Buprenorphine may mask pain from some diseases. Do not forget to advise your doctor if you take this medicine.
Children and adolescents
Due to lack of data in adolescents (age 16-18), Buprenorphine should be used only with caution in this age group.
Other medicines and Buprenorphine
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. In particular, tell your doctor if you are taking or have recently taken any of the medicines listed below as they may interact with Buprenorphine.
The following medicines have sedative effects (make you feel sleepy/drowsy). These effects are increased if these medicines are taken while you are being treated with Buprenorphine :
- benzodiazepines (used for treatment of anxiety or sleep disorders) e.g. diazepam and temazepam: you should not take these medicines while you are taking Buprenorphine , unless prescribed by your doctor because this combination can be fatal if the correct dose is not carefully determined
- strong painkillers or cough medicines containing opioid-related medicines e.g. codeine, dihydrocodeine and morphine
- medicines used for the treatment of depression, including medicines known as monoamine oxidase inhibitors (MAOI; e.g. phenelzine). Buprenorphine should not be taken at the same time or for two weeks after the end of treatment with MAOI.
- antihistamine medicines (used for treatment of allergy and/or hay fever) e.g. promethazine and chlorphenamine
- barbiturates and other medicines used for the treatment of anxiety or sleep disorders
- medicines known as antipsychotics (used for the treatment of schizophrenia) e.g. chlorpromazine and haloperidol
- certain medicines for the treatment of high blood pressure (antihypertensives) e.g. clonidine.
If you are taking any of the following medicines, your doctor may need to prescribe a lower dose of Buprenorphine:
- the antifungal medicine, ketoconazole (which can increase the levels of Buprenorphine in your blood if both are taken at the same time)
- medicines used to treat infections caused by viruses (antiviral agents) e.g. ritonavir, saquinavir and indinavir, used in the treatment of HIV infections
- oral contraceptive medicines containing gestodene
- certain medicines called ‘macrolide antibiotics' (used for the treatment of infections), e.g troleandomycin.
If you are taking any of the following medicines, your doctor may need to prescribe a higher dose of Buprenorphine :
- medicines used for the treatment of epilepsy e.g. phenobarbital, carbamazepine and phenytoin
- the antibiotic medicine, rifampicin (used for the treatment of tuberculosis)
- anticoagulant medicine, phenprocoumon (to thin your blood).
Use of Buprenorphine at the same time as the following medicines may cause withdrawal symptoms:
- methadone (used for treatment of opioid drug addiction)
- naltrexone (used to help you to remain free from your dependence on heroin, methadone and other similar opiate drugs of addiction).
Buprenorphine with food, drink and alcohol
You should not drink alcohol or take any medicines that contain alcohol while taking Buprenorphine . Alcohol increases the sedative effects of buprenorphine, which can make driving and operating machinery hazardous.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking this medicine.
Buprenorphine should only be used during pregnancy in case the potential benefits outweigh the risks. Tell your doctor if you are pregnant or trying to become pregnant. If you become pregnant during treatment with buprenorphine, tell your doctor straight away. He will decide if your treatment should be continued with an alternative medication.
Since Buprenorphine is passed into breast milk, you must not breast-feed while taking this medicine.
Driving and using machines
Buprenorphine may cause drowsiness, particularly when taken together with alcohol or certain antidepressants. If you feel drowsy while being treated with this medicine, you should not drive or operate machinery.
Buprenorphine contains lactose and butylated hydroxyanisole
Buprenorphine sublingual tablets contain lactose. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine. Buprenorphine sublingual tablets contain butylated hydroxyanisole (E320), which may cause local skin reactions (e.g. contact dermatitis), or irritation to the eyes and mucous membranes.
3. How to take Buprenophine
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Your doctor may wish to perform some tests to see how well your liver is working before starting treatment with Buprenorphine, and at regular intervals during treatment.
To avoid sudden withdrawal symptoms, treatment with Buprenorphine should be given when there are already clear signs of withdrawal symptoms.
375 mm
248 mm
|
Name of Product: Buprenorphine 2 mg/ 8 mg - sublingual tablets |
AWS TYPE |
Country |
Language |
Location | |
|
PIL |
EUUK |
ENGLISH |
Dadra | ||
|
Code |
044BP1982C |
Void artwork Code No: 044BP1982B |
CD-PDD- | ||
|
Actual Size |
248 x 375 mm |
Reason: change in text |
REMARKS: Artwork Prepared by : Sun Pharmaceutical Industries Limited Packaging Development Department [PDD] SPIL - Vadodara | ||
|
Specification / Type of paper |
No. of Colors : 1 | ||||
|
Super Fine 41 GSM Tissue Paper ITC |
Color codes: BLACK | ||||
|
Folding 375--4 zigzag --31.25 mm 248--2 --62 mm | |||||
|
Prepared by |
Checked by |
Approved by |
Approved by RA | ||
|
Approval History Attached | |||||
|
y |
\ |
|
FONTS SPECIFICATION | |
|
Patient info. Leaflet |
Swiss 721 CN BT - 11 pt |
|
Title and Tiltle Heading |
Swiss 721 CN BT - 15 pt and 13 pt |
|
Body Text |
Swiss 721 CN BT - 9 pt |
|
V_ |
_y |
100
95
75
25
5
0

Blank Area do not print here
Dosage
For adults and adolescents aged 16 years or older
The usual starting dose is between 0.8 mg to 4 mg, taken once a day. 0.4 mg dose strength of Buprenorphine is not available. If low dose is required, you should use tablets (0.4 mg) of another manufacturer.
For drug addicts who have not had any withdrawal treatment
One dose of Buprenorphine should be taken at least 4-6 hours after the last use of the opioid (narcotic such as morphine or heroin), or when the first signs of craving withdrawal appear. If you take it less than 4-6 hours after you use a narcotic you may get craving withdrawal symptoms.
For patients taking methadone
Your doctor should reduce the dose of methadone to no more than 30 mg per day before starting treatment with Buprenorphine. Buprenorphine may cause withdrawal symptoms in patients who are dependent on methadone if used within 24 hours of the last dose of methadone.
Use in children and adolescents (below 16 years)
There are no clinical data on efficacy and safety for the use of Buprenorphine in children and adolescents. Therefore, Buprenorphine should not be used by children or adolescents under 16 years old.
How to take Buprenorphine
Do not take the tablets at the same time as food or drink.
The tablets are described as ‘sublingual'. This means that the tablet should be placed under the tongue and kept there until fully dissolved, which usually occurs within 5 to 10 minutes.
Do not chew or swallow the tablets whole - the medicine will not work this way and you may get withdrawal symptoms.
How long to take Buprenorphine
During your treatment, your doctor may increase your dose of Buprenorphine up to dose of 24 mg per day, depending upon how you get on. Once you have been stable for a while, your doctor will gradually reduce your dose. With careful medical supervision, your dose may continue to be reduced until it is stopped altogether.
The effectiveness of this treatment depends on the dose and a combination of the associated medicinal, psychological and social treatment.
If you have the impression that the effect of Buprenorphine is too strong or too weak, talk to your doctor or pharmacist.
If you take more Buprenorphine than you should
Tell your doctor immediately or contact your nearest hospital casualty department. Remember to take the pack and any remaining tablets with you.
If you forget to take Buprenorphine
You should tell your doctor and follow their instructions. Do not take a double dose to make up _ for a forgotten dose, unless your doctor tells you to.
If you stop taking Buprenorphine
Do not suddenly stop taking the tablets unless told to do so by your doctor, as this may cause withdrawal symptoms.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Misusing this medicine by injecting it can cause withdrawal symptoms, infections, other skin reactions and potentially serious liver problems (see ‘Take special care with Buprenorphine ').
Drug dependence can occur as a result of taking this medicine.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Buprenorphine
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP The expiry date refers to the last day of that month.
This medicinal product does not require any special storage conditions.
Do not throw away any medicines via wastewater or house hold waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Buprenorphine contains
- The active substance is buprenorphine.
Buprenorphine 2 mg: each tablet contains 2.16 mg buprenorphine hydrochloride equivalent to 2 mg buprenorphine.
Buprenorphine 8 mg: each tablet contains 8.62 mg buprenorphine hydrochloride equivalent to 8 mg buprenorphine.
- The other ingredients are: lactose monohydrate, mannitol, citric acid anhydrous, sodium citrate dihydrate, povidone K30, butylated hydroxyanisole (E320), maize starch, maize starch pregelatinized, magnesium stearate.
What Buprenorphine looks like and contents of the pack
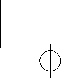
Buprenorphine 2 mg sublingual tablet: white to off-white, round, biconvex uncoated sublingual tablet debossed with “2” on one side and plain on the other side.
Buprenorphine 8 mg sublingual tablet: white to off-white, round, biconvex uncoated sublingual tablet debossed with “8” on one side and plain on the other side.
The tablets are marketed in blisters of 7 and 28 sublingual tablets.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer Marketing Authorisation Holder
Sun Pharmaceutical Industries Europe B.V.
Polarisavenue 87 2132 JH Hoofddorp The Netherlands
375 mm
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
All medicines can cause allergic reactions although serious allergic reactions are very rare. If you get any of the following symptoms after taking these tablets, you should contact your doctor immediately:
- any sudden wheeziness, difficulty in breathing or dizziness, swelling of the eyelids. face, lip or throat
- peeling and blistering of the skin, mouth, eyes and genitals
- rash affecting your whole body.
If you develop severe fatigue, loss of appetite or if your skin or eyes look yellow, tell your doctor immediately.
The following side effects have also been reported:
Manufacturer
Alkaloida Chemical Company Kabay Janos ut 29 4440 Tiszavasvari Hungary
This medicinal product is authorised in the Member States of the EEA under the following names:
Italy: Buprenorfina SUN 2 mg compresse sublinguale
Buprenorfina SUN 8 mg compresse sublinguale The Netherlands: Buprenorphine SUN 2 mg tabletten voor sublinguaal gebruik Buprenorphine SUN 8 mg tabletten voor sublinguaal gebruik United Kingdom: Buprenorphine 2 mg sublingual tablets Buprenorphine 8 mg sublingual tablets
This leaflet was last revised in 06/2015
Common: may affect up to 1 in 10 people
- difficulty in sleeping
- headache
- fainting
O
CM
CO
SI
CQ
"M"
■M-
CO
- drowsiness
- fall in blood pressure on changing position from sitting or lying down to standing
- constipation
- nausea
- vomiting
- weakness or lack of energy
- sweating.
After your first dose, you may suffer from opioid withdrawal symptoms. This is more likely if Buprenorphine is taken less than six hours after using an opioid (e.g. heroin).
Rare: may affect up to 1 in 1,000 people
- hallucinations (seeing and hearing things that are not there) and severe difficulty in breathing.
Very rare: may affect up to 1 in 10,000 people
- liver problems with or without jaundice.
CD
CD
"O
0—
o
o
o
<za
a
a
Z5
CO
248 mm
Size: 248x375 mm
100
95
75
25
5
0

Buprenorphine-2mg-8mg-248x375mm-PIL-EUUK-REV2-11-06-15
11 June 2015 09:45:25