Buprenorphine 8 Mg Sublingual Tablets
|
Sandoz Limited | |
|
Buprenorphine 0.4mg, 2mg, 8mg Sublingual Tablets |
PL 04416/0948-0950 |
PACKAGE LEAFLET: INFORMATION FOR THE USER
Buprenorphine, 0.4 mg, sublingual tablets Buprenorphine, 2 mg, sublingual tablets Buprenorphine, 8 mg, sublingual tablets
buprenorphine
Read all of this leaflet carefully before you start taking this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Buprenorphine is and what it is used for
2. Before you take Buprenorphine
3. How to take Buprenorphine
4. Possible side effects
5. How to store Buprenorphine
6. Further information
1. WHAT BUPRENORPHINE IS AND WHAT IT IS USED FOR
Buprenorphine is used to treat
• opioid dependence
Buprenorphine is part of a medical, social and psychological treatment programme for patients addicted to opiate (narcotic) drugs. Treatment is prescribed and monitored by doctors who are specialists in the treatment of drug dependence.
Treatment with Buprenorphine is intended for use in adults and adolescents aged 15 years and over.
2. BEFORE YOU TAKE BUPRENORPHINE Do not take Buprenorphine
if you are/have
• allergic (hypersensitive) to:
- buprenorphine or
- any of the other ingredients of this medicine
• serious breathing problems
• a serious liver disease
• intoxicated due to alcohol
• withdrawal symptoms, such as shakes and hallucinations
• under 15 years of age
Take special care with Buprenorphine
Inform your doctor if any of the following apply to you:
• reduced breathing function, such as asthma
You must not use Buprenorphine if you have serious breathing problems.
|
Sandoz Limited | |
|
Buprenorphine 0.4mg, 2mg, 8mg Sublingual Tablets |
PL 04416/0948-0950 |
• recent head injury or brain disease
• reduced kidney function
• reduced liver function
Acute liver problems have been reported which could be due to:
- misuse, especially through administration into a vein and at a high dose
- previous existing liver problems
- viral infections, such as hepatitis B or hepatitis C
- alcohol abuse
- anorexia
- liver damaging medicines
Inform your doctor immediately if you experience symptoms of liver problem such as severe fatigue, itching and yellow skin or eyes. You can then receive the proper treatment.
However, you must not use Buprenorphine if you have a serious liver disease.
• low blood pressure
• in men: urinary disorders, especially due to enlarged prostate
In general, all patients have to undergo a liver test prior to Buprenorphine therapy. Regular monitoring by the doctor is required if you have liver problems.
This medicinal product may mask pain of other diseases. Advise your doctor that you are taking this medicine.
Misuse and abuse
Some users died from breathing failure after misusing buprenorphine or combining it with brain acting substances, such as:
• alcohol
• medicines which calm, induce sleep or relax muscles with active substance names ending with “azepam”, such as diazepam, temazepam
• other opioids
If it is not possible for you to stop misuse, please talk to your doctor, who will provide advice on adequate treatment for problems like depression, anxiety or insomnia.
This product can cause withdrawal symptoms if you take it
• less than 6 hours after using a narcotic, such as morphine, heroin
• or less than 24 hours after you use methadone
You must not pass this medicine to others. This is forbidden and may be deadly - especially if one is not used to opiates. A dose suitable for you may be deadly for another person.
Effects of misuse for doping
Using Buprenorphine can lead to positive results in doping tests. Abusing Buprenorphine for doping purposes can endanger your health.
Taking other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
The following medicines can affect or be affected by Buprenorphine:
• medicines which calm, induce sleep or relax muscles with active substance names ending with “azepam”, such as diazepam, temazepam
|
Sandoz Limited | |
|
Buprenorphine 0.4mg, 2mg, 8mg Sublingual Tablets |
PL 04416/0948-0950 |
This combination can be fatal. Therefore, the dose must be limited. Do not take these medicines while taking Buprenorphine, where risk of misuse exists.
• medicines to treat epilepsy or to sedate, with active substance names mostly ending with “tal”, such as phenobarbital
• other medicines used to treat anxiety or sleep disorders
• other strong painkillers or medicines to treat cough, such as codeine, dihydrocodeine, morphine
• methadone, a medicine to treat severe pain, or to counter addiction
• naltrexone, a medicine to counter alcohol or opiate drug addiction
• certain medicines to treat depression or Parkinson’s disease known as monoamine oxidase inhibitors, such as moclobemide
• other medicines to treat depression
• medicines to treat allergies, sleep disturbances or cold; or prevent and treat nausea and vomiting; such as doxylamine, diphenhydramine
• medicines to treat mental or anxiety disorders, with sedative effects, such as chlorpromazine, haloperidol
• certain medicines to treat high blood pressure, such as clonidine
• Your doctor may prescribe you a lower Buprenorphine dose if you take any of the following medicines:
- ketoconazole, itraconazole: medicines to treat fungal infections
- medicines to treat HIV infections, such as ritonavir, nelfinavir, indinavir
• Your doctor may supervise you closely if you take any of the following medicines:
- medicines to treat epilepsy, such as phenobarbital, carbamazepine, phenytoin
- rifampicin: a medicine to treat bacterial infections
If you need the help of a doctor or a hospital, you must provide information on your substitution therapy and also be honest relating to your actual consumption of other pharmaceuticals or drugs. This is necessary to avoid dangerous combinations.
Taking Buprenorphine with food and drink
Avoid drinking alcohol or taking any medicines that contain alcohol while taking Buprenorphine.
Pregnancy and breast-feeding
• Pregnancy
Buprenorphine is not recommended during pregnancy. Tell your doctor before taking Buprenorphine if you are pregnant, trying or become pregnant during treatment.
• Breast-feeding
Discontinue breast-feeding while taking Buprenorphine as it will pass into your milk and may harm the breast-fed child.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Do not drive or use machines if you feel drowsy while being treated with this medicine.
Buprenorphine can impair alertness and the ability to react. Therefore, ask your doctor whether and under what circumstances you can drive a vehicle, for example.
Important information about some of the ingredients of Buprenorphine
Buprenorphine contains lactose. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine.
|
Sandoz Limited | |
|
Buprenorphine 0.4mg, 2mg, 8mg Sublingual Tablets |
PL 04416/0948-0950 |
3. HOW TO TAKE BUPRENORPHINE
Always take Buprenorphine exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
The usual dose is:
• Take the dose once a day unless otherwise prescribed by your doctor.
Your doctor will determine the best dose for you. During your treatment, the doctor may adjust the dose, depending upon your response.
If your craving for drugs is not suppressed completely, please inform your doctor.
The suitable dose is found, if you do not experience symptoms of withdrawal. However too high doses will result in sedation or drowsiness.
Patients with reduced liver function
Your doctor will probably prescribe a lower dose than those described above. However, you must not use Buprenorphine if you have serious liver diseases.
Method of use
The tablets are described as “sublingual”. This means that the tablet should be placed under the tongue and kept there until fully dissolved. This usually takes 5 to 10 minutes.
This form of use is the only effective administration route for this product.
Do not suck, chew or swallow the tablets whole - the medicine will not work this way. Do not take the tablets at the same time as food or drink.
Only sublingual application is allowed. Other forms of application (such as intravenous misuse) may lead to life-threatening intoxications with buprenorphine. In addition, excipients of the tablet and bacterial contamination may lead to health hazards such as hypersensitivity reactions, shock, inflammation of the heart, thromboembolism and sepsis.
Duration of use
This will be decided by your doctor.
After a period of successful treatment, the doctor may gradually reduce the dose to a lower maintenance dose. Depending on your condition, the Buprenorphine dose may be further reduced under careful medical supervision, eventually it may be stopped.
Do not change the treatment in any way or stop treatment without the agreement of the attending doctor.
The effectiveness of this treatment depends:
• upon the dose
• combined with the associated medical, psychological and social treatment
If you have the impression that the effect of Buprenorphine is too strong or too weak, talk to your doctor or pharmacist.
|
Sandoz Limited | |
|
Buprenorphine 0.4mg, 2mg, 8mg Sublingual Tablets |
PL 04416/0948-0950 |
Remove the sublingual tablets from the blister by pressing down on the whole tablet, so you need the least effort and you prevent the tablets from being crushed.
Instruction for use
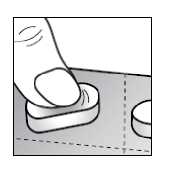
If you take more Buprenorphine than you should
Tell your doctor immediately or contact your nearest hospital casualty department. Bring the pack and any remaining tablets with you.
If you forget to take Buprenorphine
You should tell your doctor and follow his instructions. Do not take a double dose to make up a forgotten tablet, unless your doctor tells you to do so.
If you stop taking Buprenorphine
Do not suddenly stop taking the tablets unless told by your doctor, as this may cause withdrawal symptoms.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Buprenorphine can cause side effects, although not everyone gets them.
Side effects can occur with the following frequencies:
Very common, affects more than 1 per 10 users
• sleeplessness
• weakness
• withdrawal symptoms
Common, affects 1 to 10 per 100 users
• anxiety
• nervousness
• drowsiness
• dizziness
• headache, backache, stomach ache
• flow of tears
• prolonged time of the heart ventricle activity, measured with electrodes
• fainting
• fall in blood pressure when standing up quickly
• runny nose
• constipation
• diarrhoea
• nausea, vomiting
• sweating
• shiver
Uncommon, affects 1 to 10 per 1,000 users
• hallucinations
• reduced breathing function
|
Sandoz Limited | |
|
Buprenorphine 0.4mg, 2mg, 8mg Sublingual Tablets |
PL 04416/0948-0950 |
• liver cell destruction and inflammation
Very rare, affects fewer than 1 per 10,000 users
• cramps of bronchial muscles
• life-threatening allergic shock reaction
• serious allergic reaction causing swelling of the face or throat
Contact your doctor immediately if you notice any of these very rare side effects.
If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
5. HOW TO STORE BUPRENORPHINE Keep out of the reach and sight of children.
This medicinal product does not require any special storage conditions.
Do not use Buprenorphine after the expiry date, which is stated on the carton and plastic/aluminium strip after EXP. The expiry date refers to the last day of that month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION What Buprenorphine contains:
The active substance is buprenorphine as buprenorphine hydrochloride.
Buprenorphine, 0.4 mg, sublingual tablets Each tablet contains 0.4 mg buprenorphine.
Buprenorphine, 2 mg, sublingual tablets Each tablet contains 2 mg buprenorphine.
Buprenorphine, 8 mg, sublingual tablets Each tablet contains 8 mg buprenorphine.
• The other ingredients are citric acid anhydrous, lactose monohydrate, mannitol, sodium citrate, sodium stearyl fumarate, pregelatinised starch (maize).
What Buprenorphine looks like and contents of the pack
Buprenorphine, 0.4 mg, sublingual tablets
Buprenorphine 0.4 mg sublingual tablets are white to off-white, oval tablets (8.0 x 4.0 mm).
|
Sandoz Limited | |
|
Buprenorphine 0.4mg, 2mg, 8mg Sublingual Tablets |
PL 04416/0948-0950 |
Buprenorphine, 2 mg, sublingual tablets
Buprenorphine 2 mg sublingual tablets are white to off-white, oval tablets with a breaking notch on both sides (9.4 x 4.0 mm).
Buprenorphine, 8 mg, sublingual tablets
Buprenorphine 8 mg sublingual tablets are white to off-white, oval tablets with a breaking notch on both sides (13.5 mm x 6.6 mm).
Your medicine is available in plastic/aluminium strips containing 7, 10, 20, 24, 28, 30, 48, 49, 50 or 70 tablets.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Sandoz Limited Frimley Business Park,
Frimley,
Camberley,
Surrey,
GU16 7SR.
United Kingdom
Manufacturer
Salutas Pharma GmbH Otto-von-Guericke-Allee 1, 39179 Barleben Germany
This leaflet was last revised in 12/2012
PIL.0951-0953.003d 07/12/12 V003: Update SPC & PIL to brand leader PR