Butrans 20
Read all of this leaflet carefully before you start using this medicine.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours. If any of the side effects become serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Package leaflet: Information for the user
BuTrans® 20 micrograms/hour Transdermal Patch
Buprenorphine
The name of your medicine is BuTrans 20 micrograms/hour Transdermal Patch but will be referred to as BuTrans throughout this leaflet. This product is available in different strengths.
In this leaflet:
1. What BuTrans is and what it is used for
2. Before you use BuTrans
3. How to use BuTrans
4. Possible side effects
5. How to store BuTrans
6. Further information
1. WHAT BUTRANS IS AND WHAT IT IS USED FOR
BuTrans contain the active ingredient buprenorphine which belongs to a group of medicines called strong analgesics or ‘painkillers’. They have been prescribed for you by your doctor to relieve moderate, long-lasting pain that requires the use of a strong painkiller. BuTrans should not be used to relieve acute pain. BuTrans act through the skin. After application, buprenorphine passes through the skin into the blood. Each patch lasts for seven days.
2. BEFORE YOU USE BUTRANS
Do not use BuTrans:
• if you are allergic (hypersensitive) to buprenorphine or any of the other ingredients of BuTrans,
• if you have breathing problems;
• if you are addicted to drugs;
• if you are taking a type of medicine known as a monoamine oxidase inhibitor (examples include tranylcypromide, phenelzine, isocarboxazid, moclobamide and linezolid), or you have taken this type of medicine in the last two weeks;
• if you suffer from myasthenia gravis (a condition in which the muscles become weak);
• if you have previously suffered from withdrawal symptoms such as agitation, anxiety, shaking or sweating upon stopping taking alcohol.
BuTrans must not be used to treat symptoms
associated with drug withdrawal.
Take special care with BuTrans patches
Before treatment with BuTrans tell your doctor or
pharmacist:
• if you suffer from seizures, fits or convulsions;
• if you have a severe headache or feel sick due to a head injury or increased pressure in your skull (for instance due to brain disease). This is because the patches may make symptoms worse or hide the extent of a head injury;
• if you are feeling light-headed or faint;
• if you have severe liver problems;
• if you have ever been addicted to drugs;
• if you have a high temperature, as this may lead to larger quantities of the active ingredient being absorbed into the blood than normal.
If you have recently had an operation, please speak to your doctor before using these patches.
Taking other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription. If you use BuTrans with some other medicines, the effect of BuTrans or the other medicine may be changed.
• BuTrans must not be used together with a type of medicine known as a monoamine oxidase inhibitor (examples include tranylcypromide, phenelzine, isocarboxazid, moclobamide and linezolid), or if you have taken this type of medicine in the last two weeks.
• If you take some medicines such as phenobarbital or phenytoin (medicines commonly used to treat seizures, fits or convulsions), carbamazepine (a medicine to treat seizures, fits or convulsions and certain pain conditions), or rifampicin (a medicine to treat tuberculosis) the effects of BuTrans may be reduced.
• BuTrans may make some people feel drowsy, sick or faint or make them breathe more slowly or weakly. These side effects may be made worse if other medicines that produce the same effects are taken at the same time. These include certain medicines to treat depression, anxiety, psychiatric or mental disorders, medicines to help you sleep, medicines to treat high blood pressure such as clonidine, other opioids (which may be found in painkillers or certain cough mixtures e.g. morphine, dextropropoxyphene, codeine, dextromethorphan, noscapine), antihistamines which make you drowsy, or anaesthetics such as halothane.
• BuTrans must not be used together with benzodiazepines (medicines used to treat anxiety or to help you sleep). This combination may cause serious breathing problems which may be fatal.
Using BuTrans with alcohol
Alcohol may make some of the side effects worse and you may feel unwell if you drink alcohol whilst wearing BuTrans. Drinking alcohol whilst using BuTrans may also affect your reaction time.
Pregnancy and breastfeeding
You should not use BuTrans if you are pregnant, likely to become pregnant or are breastfeeding.
Ask your doctor or pharmacist for advice before taking any medicines.
Driving and using machines BuTrans may affect your reactions to such an extent that you may not react adequately or quickly enough in the event of unexpected or sudden occurrences. This applies particularly:
• at the beginning of treatment;
• if you are taking medicines to treat anxiety or help you sleep;
• if your dose is increased.
If you are affected you should not drive or operate machinery whilst using BuTrans, or for 24 hours after removing the patch.
The medicine can affect your ability to drive as it may make you sleepy or dizzy.
• Do not drive while taking this medicine until you know how it affects you.
• It is an offence to drive while you have this medicine in your body over a specified limit unless you have a defence (called the ‘statutory defence’).
• This defence applies when:
o The medicine has been prescribed to treat a medical or dental problem; and o You have taken it according to the instructions given by the prescriber and in the information provided with the medicine.
• Please note that it is still an offence to drive if you are unfit because of the medicine (i.e. your ability to drive is being affected).
Details regarding a new driving offence concerning driving after drugs have been taken in the UK may be found here:

https://www.gov.uk/drua-driving-law
Talk to your doctor or pharmacist if you are not sure
whether it is safe for you to drive while taking this
medicine.
3. HOW TO USE BUTRANS
Three different strengths of BuTrans are available. Your doctor will decide which strength of BuTrans patch will suit you best. During treatment, your doctor may change the patch you use to a smaller or larger one if necessary. You should not apply more than two patches at the same time, regardless of the patch strength.
Always use the BuTrans patch exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Adults and elderly patients
Unless your doctor has told you differently, attach one BuTrans patch (as described in detail below) and change it every seventh day, preferably at the same time of day. Your doctor may wish to adjust the dose after 3-7 days until the correct level of pain control has been found. If your doctor has advised you to take other painkillers in addition to the patch, strictly follow the doctor’s instructions, otherwise you will not fully benefit from treatment with the BuTrans patch. The patch should be worn for 3 full days before increasing the dose, this is when the maximum effect of a given dose is established.
Patients under 18 years of age
BuTrans should not be used in patients below the age
of 18 years.
Patients with kidney disease/dialysis patients
In patients with kidney disease, no change in dose is necessary.
Patients with liver disease
In patients with liver disease, the effects and period of action of the BuTrans patch may be affected and your doctor will therefore check on you more closely.
Before applying the BuTrans patch
• Choose an area of non-irritated, intact skin on your upper arm, outer arm, upper chest, upper back or side of the chest. (See illustrations below). Ask for assistance if you cannot apply the patch yourself.
• The BuTrans patch should be applied to a relatively hairless or nearly hairless skin site. If no suitable hair free sites are available the hairs should be cut off with a pair of scissors. Do not shave them off.
• Avoid skin which is red, irritated or has any other blemishes, for instance large scars.
• The area of skin you choose must be dry and clean. If necessary, wash it with cold or lukewarm water. Do not use soap, alcohol, oil, lotions or other detergents. After a hot bath or shower, wait until your skin is completely dry and cool. Do not apply lotion, cream or ointment to the chosen area. This might prevent your patch from sticking properly.
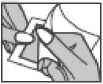
Applying the patch
Step 1: Each patch is sealed in a pouch. Just before use, open the pouch by tearing where indicated. Take out the patch. Do not use the patch if the pouch seal is broken.
Step 2: The sticky side of the patch is covered with a silvery protective foil. Carefully peel off half the foil. Try not to touch the sticky part of the patch.
Step 4: Press the patch against your skin with the palm of your hand and count slowly to 30. Make sure that the whole patch is in contact with your skin, especially at the edges.
Wearing the patch
You should wear the patch for seven days. Provided that you have applied the patch correctly, there is little risk of it coming off. If the edges of the patch begin to peel off, they may be taped down with a suitable skin tape. You may shower, bathe or swim whilst wearing it.
Do not expose the patch to extreme heat (e.g. heating pads, electric blanket, heat lamps, sauna, hot tubs, heated water beds, hot water bottle, etc) as this may lead to larger quantities of the active ingredient being absorbed into the blood than normal. External heat may also prevent the patch from sticking properly.
If you have a high temperature this may alter the effects of BuTrans (see “Take special care” section above).
In the unlikely event that your patch falls off before it needs changing, do not use the same patch again. Stick a new one on straight away (see “Changing the patch” below).
Changing the patch
• Take the old patch off.
• Fold it in half with the sticky side inwards.
• Open and take out a new patch. Use the empty pouch to dispose of the old patch. Now discard the pouch safely.
• Even used patches contain some active ingredient that may harm children or animals, so make sure your used patches are always kept out of the reach and sight of them.
• Stick a new patch on a different appropriate skin site (as described above). You should not apply a new patch to the same site for 3-4 weeks.
• Remember to change your patch at the same time of day. It is important that you make a note of the time of day.
Duration of treatment
Your doctor will tell you how long you should be treated with the BuTrans patch. Do not stop treatment without consulting a doctor, because your pain may return and you may feel unwell (see also “If you stop using BuTrans" below).
If you feel that the effect of the BuTrans patch is too weak or too strong, talk to your doctor or pharmacist.
If you use more BuTrans patches than you should
As soon as you discover that you have used more patches than you should, remove all patches and call your doctor or hospital straight away. People who have taken an overdose may feel very sleepy and sick. They may also have breathing difficulties or lose consciousness and may need emergency treatment in hospital.
When seeking medical attention make sure that you take this leaflet and any remaining patches with you to show to the doctor.
If you forget to apply the BuTrans patch
Stick a new patch on as soon as you remember. Also make a note of the date, as your usual day of changing may now be different. If you are very late changing your patch, your pain may return. In this case, please contact your doctor.
Do not apply additional patches to make up for the forgotten application.
If you stop using BuTrans patches
If you stop using BuTrans too soon or you interrupt your treatment your pain may return. If you wish to stop treatment please consult your doctor.
They will tell you what can be done and whether you can be treated with other medicines.
Step 3: Stick the patch on to the area of skin you have chosen and remove the remaining foil.

POM
Some people may have side effects when they have used strong painkillers for a long time and stop using them. The risk of having effects after stopping BuTrans is very low. However, if you feel agitated, anxious, nervous or shaky, if you are overactive, have difficulty sleeping or digestive problems, tell your doctor.
The pain relieving effect of BuTrans patch is maintained for some time after removal of the patch. You should not start another opioid analgesic (strong painkiller) within 24 hours after removal of the patch.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines BuTrans can have side effects, although not everybody gets them.
Serious side effects that may be associated with BuTrans are similar to those seen with other strong painkillers and include difficulty in breathing and low blood pressure.
This medicine can cause allergic reactions, although serious allergic reactions are rare. Remove the patch and tell your doctor immediately if you get any sudden wheeziness, difficulties in breathing, swelling of the eyelids, face or lips, rash or itching especially those covering your whole body.
As with all strong painkillers, there is a risk that you may become addicted or reliant on BuTrans.
In patients treated with BuTrans, the following other side effects have been reported:
Very common (probably occurring in more than 1 in 10 people)
• Headache, dizziness, drowsiness.
• Constipation, dry mouth, feeling or actually being sick.
• Itching, redness, itching at application site.
Common (probably occurring in between 1 and 10 out of every 100 people)
• Loss of appetite.
• Confusion, depression, difficulty in sleeping, nervousness.
• Tingling or numbness.
• Flushing of the skin.
• Shortness of breath.
• Abdominal pain or discomfort, diarrhoea, indigestion.
• Sweating, rash, skin eruptions.
• Tiredness, a feeling of unusual weakness, muscle weakness, pain, chest pain, swelling of hands, ankles or feet, redness or rash at the application site.
Uncommon (probably occurring in between 1 and 10 out of every 1,000 people)
• Dehydration.
• Mood swings, restlessness, agitation, anxiety, feeling detached from oneself, a feeling of extreme happiness, hallucinations, nightmares.
• Changes in taste, difficulty in speaking, reduced sensitivity to pain or touch, loss of memory, migraine, fainting, shaking, problems with concentration or coordination.
• Dry eyes, blurred vision.
• A ringing or buzzing sound in the ears, a feeling of dizziness or spinning.
• High or low blood pressure, angina (severe chest pain associated with heart disease), fast or irregular heartbeat.
• Worsening of breathing problems associated with asthma, cough, hiccups, over breathing, reduced oxygen in the blood, runny nose, wheezing.
• Wind.
• Weight loss.
• Dry skin, swelling of the face.
• Muscle cramps, spasms, aches and pains.
• Difficulty in passing urine.
• A flu like illness, high temperature, shivering, generally feeling unwell.
• An increase in accidental injuries (e.g. falls).
• Withdrawal symptoms such as agitation, anxiousness, sweating or shaking upon stopping using BuTrans
If you need to have blood tests remind your doctor that you are using BuTrans. This is important because BuTrans may change the way your liver works and this could affect the results of some blood tests.
Rare (probably occurring in between 1 and 10 out of every 10,000 people)
• Decreased sexual drive, mental disorder.
• Difficulties with balance.
• Swelling of the eyelids, a reduction in size of the pupils in the eye.
• Difficulty in breathing.
• Diverticulitis (inflammation of the intestine), difficulty in swallowing.
• Local allergic reaction with marked signs of swelling (in such cases treatment should be stopped).
• Decreased erection, sexual dysfunction.
Very rare (probably occurring in fewer than 1 out of every 10,000 people)
• Muscle twitching.
• Ear pain.
• Blisters.
If any of the side effects become serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE BUTRANS
Keep out of the reach and sight of children.
Do not use BuTrans after the expiry date which is stated on the carton and on the pouch. The expiry date refers to the last day of that month. After the expiry date, take any unused patches to a pharmacy.
Do not store BuTrans above 25°C.
Do not use the patch if the pouch seal is broken.
Used patches must be folded over on themselves with the adhesive layer inwards, and discarded safely out of sight and reach of children.
6. FURTHER INFORMATION
What BuTrans contains:
One transdermal patch (active surface area: 25cm2) contains 20 mg of buprenorphine and releases 20 micrograms/hour over a period of 7 days.
The other ingredients are:
• Levulinic Acid
• Oleyl Oleate
• Povidone K90
• Polyacrylate (Durotak 387-2051 & 387-2054)
• Polyethylene terephthalate (foil/backing layer/liner)
What BuTrans looks like and contents of the pack
Beige square patches with rounded corners marked with Norspan 20pg/h in black ink.
Manufacturer
BuTrans Transdermal Patches are manufactured by Bard Pharmaceuticals Limited, Cambridge Science Park, Milton Road, Cambridge CB4 0GW, UK.
Procured from within the EU and repackaged in the UK by the Parallel Import Product Licence holder: CD Pharma Ltd, Unit 3 Manor Point, Manor Way, Borehamwood, Herts WD6 1 EE.
BuTrans 20 micrograms/hour Transdermal Patch PL 20492/0525
BuTrans is a registered trademark. Date of preparation: 14th April 2016