Canesten Thrush Combi Internal & External Creams 10% W/W / 2% W/W Vaginal Cream & Cream

|
Trident Reference No: |
BAY146110 |
|
Bayer Ref No: |
TR777636 |
|
Brand: |
Canesten |
|
Range: |
Combi |
|
Product: |
Internal and External Creams |
|
Pack Type: |
leaflet |
|
Pack Size: |
LeafletN/A |
|
Action: |
E |
|
Date: |
30/07/14 |
|
Country: |
UK |
|
Finished Goods Code: |
80728042 |
|
Synaps Request Code: |
TL012031 |
|
Packaging Comp Code: 8094122-13 | |
|
CAD Ref No: |
PIL 174 X 297 folded 174X 38 (P039) |
|
Printer: |
Generic Bayer |
|
Packaging Site: |
Kem Pharma |
|
Substrate: |
White Paper |
|
Barcode Type: |
N/A |
|
Magnification: |
N/A |
|
Pharmacode No/NE: |
0100000(158) |
|
Font Size | |
|
(Smallest on artwork): |
9.00pt |
|
Technical ft Non Printing Items | |
|
| Cutter | Guides | |
Colours i

E3
TRIDENT
Connaught House, Connaught Road, Kingswood Business Park, Hull, HU7 3AP, England. T: +44 (0) 1482 828100
Please note that any low resolution paper Canon colour copies associated with this job should he referred to for content, layout and colour separation only.
UNDER NO CIRCUMSTANCES SHOULD THIS ARTWORK BE ALTERED WITHOUT PRIOR PERMISSION FROM TRIDENT.

r
[Read all of this leaflet carefully because it contains important information for you.
[This medicine is available without prescription. However, you [still need to use Canesten Thrush Combi Internal & External Creams carefully to get the best results from it. i* Keep this leaflet. You may need to read it again, j* Ask your pharmacist if you need more information or advice. [* You must contact a doctor if your symptoms worsen or do ' not improve in 7 days.
i* If you have any unusual effects after using this product,
[ tell your doctor or pharmacist.
|IN THIS LEAFLET
n. What is Canesten Thrush Combi and what is it used for?
■2. Before you use Canesten Thrush Combi [5. How to use Canesten Thrush Combi [4. Possible side effects [5. How to store Canesten Thrush Combi 6. Further information
1. WHAT IS CANESTEN® THRUSH
; COMBI AND WHAT IS IT USED FOR?
Canesten Thrush Combi Internal & External Creams is a [complete treatment for vaginal thrush because it treats both [the internal cause and external symptoms.
[Only use this product if you have been previously [diagnosed by your doctor as having vaginal thrush.
[The active substance in Canesten Thrush Combi Internal & [External Creams is clotrimazole. Clotrimazole belongs to a [group of medicines called azoles and is an antifungal agent which fights the cause of infections such as vaginal thrush.
2. BEFORE YOU USE CANESTEN®
; THRUSH COMBI
1D0 not use Canesten* Thrush Combi Internal & External [Creams:
j* If you are allergic (hypersensitive) to clotrimazole or any of 1 the other ingredients, including cetostearyl alcohol, of 1 Canesten Thrush Combi Internal & External Creams 1 (see Section 6. Further Information).
[• During your period as it may be less effective.
[* To treat nail and scalp infections.
[Before using Canesten* Thrush Combi Internal & [External Creams, you should see your doctor if:
[• You are unsure whether you have thrush or this is the first [ time you have had these symptoms.
* You have had more than two infections of thrush in the last 1 six months.
[• You or your partner have ever had a sexually transmitted [ disease.
i* You are aged under 16 or over 60.
1* You have ever had an allergic reaction to Canesten or any [ other vaginal antifungal products.
[• You have any of the following symptoms:
1 * Irregular vaginal bleeding.
1 * Abnormal vaginal bleeding or a blood-stained
[ discharge.
[ * Ulcers, blisters or sores of the vagina or vulva.
1 • Lower abdominal pain.
1 * Pain or difficulty in passing urine.
I • Fever or chills.
[ * Feeling sick or vomiting.
[ * Diarrhoea.
1 * A foul smelling discharge from the vagina.
Important information about some of the ingredients:
The creams contain cetostearyl alcohol which may cause local skin irritation (e.g. rash, itching or redness).
Special precautions:
As with other creams, this product may reduce the effectiveness of rubber contraceptives, such as condoms or diaphragms. Consequently, you should use alternative precautions for at least five days after using this product.
Do not use tampons, intravaginal douches, spermicides or other vaginal products while using this product.
Avoid vaginal intercourse while you have thrush and during use of this product because your partner could become infected.
Using other medicines:
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Inform your doctor if you are taking tacrolimus or sirolimus (used to reduce the immune response to prevent rejection after an organ transplant).
Pregnancy and breast-feeding:
If you are pregnant, breast-feeding or trying for a baby, tell your doctor or midwife before using Canesten Thrush Combi Internal & External Creams. If you have informed your doctor or midwife already, follow his/her instructions carefully.
To treat internal thrush, your doctor may recommend that you use a treatment that can be inserted in the vagina without the help of an applicator, such as a clotrimazole pessary
3. HOW TO USE CANESTEN®
THRUSH COMBI
The Internal Cream:
The cream should be inserted as high as possible into the vagina, preferably before going to sleep at night for convenient and comfortable treatment.
Wash your hands before removing the foil from the blister pack and again afterwards when you have used the applicator.
1. Remove the applicator from the packaging. Keeping the red cap in place, insert the tip of plunger A into the applicator B (approximately 1cm).
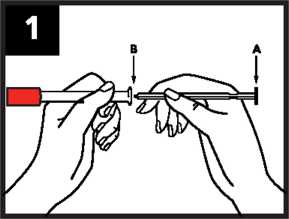
2. Twist and pull to remove the red cap C. Be careful not to press the plunger in any further before you have inserted the applicator into the vagina. This will avoid wasting any cream.
2
[This is because Canesten Thrush Combi Internal & External [Creams may not be the right treatment for you.
c
Packaging Site Approval:
|
Name: | |
|
Date: | |
|
Action: |
Country Approval:
|
Name: | |
|
Date: | |
|
Action: |
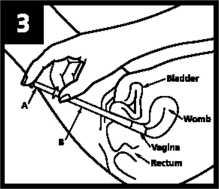
S.HareTully put the applicator iasdeepas is comfortabTelnto ithe vagina (this is easiest when lying on your back with your [knees bent up). Holding the applicator in place, slowly press [the plunger until it stops so that the pre-measured dose of ■cream is deposited into the vagina.
|
Trident Reference No: |
BAY146110 |
|
Bayer Ref No: |
TR777636 |
|
Brand: |
Canesten |
|
Range: |
Combi |
|
Product: |
Internal and External Creams |
|
Pack Type: |
leaflet |
|
Pack Size: |
LeafletN/A |
|
Action: |
E |
|
Date: |
30/07/14 |
|
Country: |
UK |
|
Finished Goods Code: |
80728042 |
|
Synaps Request Code: |
TL012031 |
|
Packaging Comp Code: 8094122-13 | |
|
CAD Ref No: |
PIL 174 X 297 folded 174X 38 (P039) |
|
Printer: |
Generic Bayer |
|
Packaging Site: |
Kem Pharma |
|
Substrate: |
White Paper |
|
Barcode Type: |
N/A |
|
Magnification: |
N/A |
|
Pharmacode No/NE: |
0100000(158) |
|
Font Size | |
|
(Smallest on artwork): |
9.00pt |
|
Technical ft Non Printing Items | |
|
| Cutter | Guides | |
Colours

E3
TRIDENT
Connaught House, Connaught Road, Kingswood Business Baric, Hull, HU7 3AP, England. T: +44 (0) 1482 828100
Please note that any low resolution paper Canon colour copies associated with this job should he referred to for content, layout and colour separation only.
UNDER NO CIRCUMSTANCES SHOULD THIS ARTWORK BE ALTERED WITHOUT PRIOR PERMISSION FROM TRIDENT.

■u
•6
2
D
E
E
s
|4. Remove the applicator. Dispose of the applicator in a safe [place, out of the reach of children. The applicator cannot be [flushed down the toilet.
[The cream is deposited in the vagina, but it is quite common [to notice a slight discharge after using the cream and therefore it may be helpful to wear a parity liner. This does not mean ithat the treatment has not worked.
[The External Cream:
■Before use, pierce the tube seal by inverting the cap over the [end of the tube and press.
[To treat the itching and soreness of the vulva (vulvitis), the [cream should be thinly and evenly applied to the area around the ■entrance of the vagina, 2 or 3 times a day and smoothed in gently.
[The symptoms of thrush should disappear within three days of ■treatment. If no improvement is seen after seven days you must tell your doctor. If the infection returns after seven days [you may use one further treatment, but if you have more than [two infections within six months you should see your doctor.
[The internal cream is for use in the vagina only and [the external cream is for external use only:
[Do not put the creams in your mouth or swallow them.
[If the creams are swallowed accidentally, tell your doctor straight away or contact the Accident and Emergency [Department of your nearest hospital.
^.POSSIBLE SIDE EFFECTS
[Like all medicines, Canesten Thrush Combi Internal & External Creams can cause side effects, although not everybody gets [them. As with all medicines, some people may be allergic to the [creams. If you are allergic, a reaction will occur soon after you Jiave used the medicine. If you experience an allergic reaction or ■the redness, burning, pain, itching or swelling get worse, stop using this product and tell your doctor straight away or contact [the Accident and Emergency Department of your nearest ^ospital. Signs of an allergic reaction may include:
[• Rash.
i* Swallowing or breathing problems.
|* Swelling of your lips, face, throat or tongue.
[* Weakness, feeling dizzy or faint.
[• Nausea.
After you apply Canesten Internal Cream you might experience: !• Itching, rash, swelling, redness, discomfort, burning, irritation,
[ vaginal peeling or bleeding.
[* Pain in the abdomen or pelvic area.
After you apply Canesten External Cream you might experience: !• Itching, rash, blisters, burning, redness, discomfort, swelling, [ irritation or peeling of skin.
[If you experience any of the above effects, tell your doctor or [pharmacist immediately.
[Reporting of side effects
[If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow [Card Scheme at: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information [on the safety of this medicine.
[5.HOWTOSTORE CANESTEN®
[ THRUSH COMBI
[Keep this medicine out of the sight and reach ■of children.
Do not store above 25°CT Do not usetfie-crea ms after the ”" expiry date which is stated at one end of the carton, on the end of the tube of cream, on the applicator and on the applicator foil wrapping. The expiry date refers to the last day of that month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment
6.FURTHER INFORMATION
What Canesten® Thrush Combi Internal & External Creams contains:
Applicator of internal cream:
* The active substance is dotrimazole at a strength of 10% w/w.
* The other ingredients are benzyl alcohol, polysorbate 60, sorbitan stearate, cetostearyl alcohol, isopropyl myristate, cetyl palmitate and purified water.
External cream:
* The active substance is dotrimazole at a strength of 2% w/w.
* The other ingredients are benzyl alcohol, polysorbate 60, sorbitan stearate, cetyl palmitate, cetostearyl alcohol, octyldodecanol and purified waiter.
See Section 2 'Do not use and 'important information about some of the ingredients’for cetostearyl alcohol advice.
What Canesten9 Thrush Combi Internal & External Creams looks like and contents of the pack:
Canesten0 Thrush Combi Internal & External Creams contains a full course of treatment, which consists of a pre-filled applicator containing 5g of white internal cream and one 10g tube of white external cream.
Marketing Authorisation Holder:
Bayer pic, Consumer Care Division, Bayer House,
Strawberry Hill, Newbury, Berkshire RGl4 iJA, UK. Manufacturer:
Kern Pharma S.L., Poligon Industrial Colon II, Calle Venus 72, 08228 Terrassa, Barcelona, Spain.
Remember: If you have any doubts about using Canesten9 Thrush Combi Internal & External Creams correctly, seek the advice of your doctor or pharmacist.
Further information about vaginal thrush:
Vaginal thrush (candidiasis) is a common infection that most women suffer from at some time in their lives and is not caused by lack of personal hygiene.
Thrush is caused by a yeast (fungus) called Candida which lives harmlessly in the vagina and other parts of the body, without you even noticing it. However, the natural balance that keeps Candida under control can be upset by many factors such as hormonal changes (menstruation, contraceptive pill, pregnancy, menopause), poor health, antibiotics, perfumed soaps, bath additives and tight clothing.
If the natural pH balance is altered, the level of yeast increases and can develop into a thrush infection causing any of the following symptoms: persistent burning and/or itching around the vagina and vulva, redness, swelling and soreness of the tissues of the vagina and vulva and a whitish, odourless discharge from the vagina. Not everybody who has thrush has all these symptoms; you may have only one of them.
How to avoid future recurrences:
✓ Wear cotton knickers and loose clothing. ✓ Wash daily.
/ After going to the toilet, wipe yourself from the front to back as a thrush infection may be transferred from the bowel.
/ Change your sanitary protection regularly.
X Try to avoid wearing tights, nylon knickers and close fitting jeans.
X Try to avoid washing with perfumed soaps or using vaginal deodorants.
X Do not wash or mb yourself hard with sponges or flannels and avoid hot baths with strong perfumed oils.
If you are still worried or have any questions about the symptoms or the treatment of thrush, do not hesitate to ask your doctor or pharmacist for advice.
For UK residents only: if you have any questions or would like more information, call our Canesten Advice Line on 0845 758 5030.Calls charged at local rate. 8094122-is
This leaflet was last revised in June 2014. Bayer
Canesten is a registered trademark of Bayer AG, Germany.
Packaging Site Approval:
|
Name: | |
|
Date: | |
|
Action: |
Country Approval:
|
Name: | |
|
Date: | |
|
Action: |