Capsorin 100 Mg/Ml Oral Solution
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Capsorin 100 mg/ml oral solution.
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Oral solution containing 100 mg of ciclosporin per ml (microemulsion).
Each ml contains 100.00 mg of Ethanol, anhydrous.
For a full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Oral solution.
Clear and transparent solution.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Transplant
Organ transplantation
Prevention of graft rejection following kidney, liver, heart, combined heart-lung, lung or pancreas transplants.
Treatment of transplant rejection in patients previously receiving other immunosuppressive agents.
Bone marrow transplantation
Prevention of graft rejection following bone marrow transplantation.
Prevention or treatment of graft-versus-host disease (GVHD).
Other indications Nephrotic syndrome
Adults and children with steroid dependent or steroid resistant nephrotic syndrome due to glomerular disorders such as glomerular minimum changes, focal segmental glomerulosclerosis or membranous glomerulonephritis. It can also be used to maintain steroid-induced remission, allowing to suspend steroids.
Rheumatoid arthritis
Indicated for the treatment of severe, active rheumatoid arthritis in patients in whom classical, disease modifying anti-rheumatic drugs (DMARD’s) are inappropriate or ineffective.
Psoriasis
Treatment of severe forms of psoriasis in patients in whom conventional therapy is inappropriate or ineffective.
Atopic dermatitis
Capsorin is indicated in patients with severe atopic dermatitis in whom conventional therapy is ineffective.
4.2 Posology and method of administration Oral administration.
The daily dose of Capsorin should always be divided in 2 doses.
In transplant patients routine monitoring of the ciclosporin blood levels should be performed in order to avoid the risk of adverse reactions (if blood levels are too high) and organ rejection (if blood levels are too low).
Due to possible differences in bioavailability patients should not be transferred to or from other oral formulations of ciclosporin without appropriate close monitoring of ciclosporin blood concentrations, serum creatinine levels and blood pressure. For this reason it may be appropriate to prescribe by brand.
For drug level monitoring, whole blood is preferred, measured by a specific analytical method. A number of methods that measure unaltered ciclosporin (HPLC, specific monoclonal specific radioimmunoassays) as well as unspecific methods that also measure some metabolites have been developed for determining ciclosporin levels: The results of the various determination methods are not interchangeable. Determination of ciclosporin levels by means of specific monoclonal antibodies or by HPLC is to be given preference. Target concentration ranges depend on organ type, time after transplantation and immunosuppressive regimen.
It should be noted that other factors besides ciclosporin blood level can affect the clinical condition of the patient. The results are therefore intended only as a guide for dosing and should be used together with other clinical and laboratory parameters.
A higher oral dose of ciclosporin or an intravenous dose may be necessary if absorption is impaired by gastrointestinal disturbances.
Organ transplantation
Treatment with ciclosporin is initiated within 12 hours prior to surgery with a dose of 10-15 mg/kg given in two divided doses. This daily dose is continued 1-2 weeks following surgery whereupon the daily dose is gradually reduced in agreement with the blood concentration to a maintenance dose of approximately 2-6 mg/kg given in two divided doses.
When ciclosporin is used with other immunosuppressants (e.g. with corticosteroids or in poly- therapy), a lower dose is administered (e.g. initially 3-6 mg/kg given in 2 divided doses).
Bone marrow transplantation
For the prevention of graft-versus-host-disease (GVHD), ciclosporin is commonly used initially, short-term, in combination with methotrexate. The optimum dose should be adjusted individually. In general, treatment should be initiated 1 to 2 days before bone marrow transplantation with intravenous ciclosporin (dosage 2.5 to 5 mg/kg/day). This is replaced by oral administration as soon as patients are able to tolerate oral medication (generally at 12.5 mg/kg/day). Oral treatment should be continued for at least 3-6 months, before gradual dose reduction and eventual discontinuation.
Alternative treatment regimes are intravenous ciclosporin as mono therapy at 5 mg/kg/day (day 1 to day 3) and 3 mg/kg/day (day 4 to day 14) or combination therapy with intravenous ciclosporin at 3-5 mg/kg/day and corticosteroids. In these cases, treatment should also be changed to the oral route as soon as possible and continued over a longer period.
If Capsorin is used to initiate therapy, the recommended dose is 12.5 to 15 mg/kg/day given in 2 divided doses, starting on the day before transplantation.
Some patients may experience GVHD after discontinuation of ciclosporin treatment, but usually respond positively to repeated treatment. A low dose of ciclosporin can be used for mild, chronic GVHD.
Nephrotic syndrome
For induction of remission the recommended oral dose is 5 mg/kg/day given in two divided doses for adults and 6 mg/kg/day for children, if renal function is normal. In patients with reduced renal function, the initial dose should not exceed 2.5 mg/kg/day.
Appropriate monitoring of the ciclosporin level pre-dose to avoid overdose in children is recommended.
In focal segmental glomerulosclerosis, a combination of Ciclosporin and corticosteroids may be of benefit.
In the absence of efficacy after 3 months treatment for minimal change and focal segmental glomerulosclerosis or 6 months treatment for membranous glomerulonephritis, Capsorin therapy should be discontinued.
The dose should be individually adjusted according to efficacy (proteinuria) and safety (mainly serum creatinine), but should not exceed 5 mg/kg/day for adults and 6 mg/kg/day for children.
In maintenance treatment the dose is slowly reduced to the lowest therapeutically effective level.
Rheumatoid arthritis:
For the first 6 weeks of therapy, the recommended dose is 2.5 mg/kg/day, given in 2 divided doses. The dose may be decreased depending on tolerance. The daily dose may be increased gradually, if the clinical effect is considered insufficient. Normally, the daily dose may not exceed 4 mg/kg/day. In individual cases, the dose may be increased up to 5 mg/kg/day. If the dose is increased too soon, there is a risk of overdosage.
For maintenance, the dosage should be adjusted individually to the lowest effective dose.
Low-dose corticosteroids and/or non-steroidal anti-inflammatory drugs can be used in combination with ciclosporin (see also “4.5 Interaction with other medicinal products and other forms of interaction”).
Psoriasis:
Treatment of this condition is individually adjusted since the disease varies greatly.
For induction of remission, the recommended initial dose is 2.5 mg/kg/day orally given in 2 divided doses. If no improvement is seen after 1 month, the daily dose can gradually be increased to maximum 5 mg/kg. The treatment should be discontinued in patients with psoriasis lesions which do not show a sufficient response within 6 weeks at 5 mg/kg/day, or where the clinically effective dose is not compatible with the established safety guidelines.
An initial dose of 5 mg/kg/day is justified in patients whose condition requires rapid improvement. When a satisfactory response is achieved, treatment with Capsorin can be discontinued and a possible relapse can be treated with Capsorin at the previous clinically effective dose. Some patients may require continuous maintenance treatment.
In maintenance treatment the dose is individually titrated to the lowest clinically effective level and the dose should not exceed 5 mg/kg/day given in two divided doses.
Atopic dermatitis:
Treatment of this condition is individually adjusted, since the disease varies greatly.
The recommended dose is 2.5-5 mg/kg/day orally given in 2 divided doses, for a maximum of 8 weeks. If an initial dose of 2.5 mg/kg/day does not give a satisfactory result within two weeks, the daily dose can be increased to maximum of 5 mg/kg. In very severe cases, the disease can be controlled with an initial dose of 5 mg/kg per day. When a satisfactory response is achieved, the dose should be gradually reduced and treatment discontinued.
Administration method
The dose range is intended only as a guide Routine monitoring of ciclosporin blood level is required in order to achieve the optimal therapeutic concentration for individual patients. Monitoring can be done by means of a RIA method based on monoclonal antibodies.
The total daily dose should always be administered in tow divided doses. The divided doses should always be administered at the same time of day and the times between single doses should be approximately equivalent. Therefore, it is recommended to take the two divided doses in the morning and in the evening.
Capsorin can be administered with food or alone.
Switching from other oral ciclosporin preparations to Capsorin:
In order to switch patients from other oral ciclosporin preparations to Capsorin, ciclosporin trough blood levels, serum creatinine levels, and blood pressure should be checked prior to the switch (i.e., while using other oral ciclosporin preparations). The patient should be switched to the same daily dose of Capsorin that was used for the prior ciclosporin preparation (mg per mg conversion). It is recommended that ciclosporin trough levels, serum creatinine, and blood pressure be checked after 4 - 7 days. If necessary, the dose of Capsorin should be adjusted accordingly. Additional check-ups may be necessary in the first two months following the switch (e. g., weeks 2, 4, and 8) and the dose adjusted accordingly.
The Oral Solution should be diluted preferentially with orange juice or apple juice. However, it may also be taken with others non-alcoholic beverages, according to individual taste. The oral solution should be stirred well immediately before being taken.
Owing to its possible interference with the P450-dependent enzyme system, grapefruit juice should not be used as a diluent. The measuring device should not come into contact with the diluent. The measuring device should not be rinsed with water, alcohol or any other liquid. If it is necessary to clean the measuring device, the outside should be wiped with a dry tissue. (see section 6.6)
Dosage in renal insufficiency:
Specific investigations have not been performed on the pharmacokinetics of ciclosporin in transplant patients with impaired renal function. Special caution is required if a rapid rise in serum creatinine occurs (even within the normal range) after starting treatment with Capsorin. A rise in serum creatinine or fall in creatinine clearance may also be the expression of an acute rejection reaction, particularly after renal transplantation. Initiation of treatment with Capsorin in existing renal dysfunction and subsequent dose adjustment should only be undertaken after careful consideration of the benefits and risks, taking into account the overall clinical picture and ciclosporin blood levels.
For patients with nephrotoxic syndrome and moderately impaired renal function (baseline values of serum creatinine in adults <200 pmol/L, in children <140 pmol/L), an initial dose of 2.5 mg ciclosporin/kg body weight per day should not be exceeded. Patients must be monitored closely.
Dosage in impaired hepatic function:
Impaired liver function may considerably modify the pharmacokinetics of ciclosporin in some cases. Blood concentrations of ciclosporin (cmin) must be monitored closely in patients with impaired hepatic function and the dose adjusted accordingly.
In psoriasis, administration of Capsorin should be terminated if liver enzymes and bilirubin levels are twice the baseline values.
In nephrotic syndrome patients with severe liver function disturbances, the initial dose should be decreased by 25% to 50%.
Elderly:
There is limited experience with the use of ciclosporin in the elderly, but no special problems have been seen at the recommended dose. However, factors associated with ageing, such as impaired renal function, necessitate careful supervision and possible dosage adjustment.
Children:
Experience in children is limited. However, ciclosporin has been used at the recommended dose for children from 1 year without special problems. In several studies children needed a higher dose of ciclosporin per kg body weight than adults and they tolerated the higher dose although at dosages above the upper end of the recommended range children seem to be more susceptible to fluid retention, convulsions and hypertension. This responds to dosage reduction.
4.3 Contraindications
• Hypersensitivity to the active substance or to any of the excipients.
• Capsorin is contra-indicated in psoriatic and atopic dermatitis patients with abnormal renal function, uncontrolled hypertension, uncontrolled infections or any kind of malignancy other than that of the skin (see section 4.4 Precautions).
• Capsorin is contra-indicated in rheumatoid arthritis patients with abnormal renal function, uncontrolled hypertension, uncontrolled infections or any kind of malignancy
• Renal function disorders except in patients with nephrotic syndrome and mil-moderate renal insufficiency
• Capsorin is contra-indicated in psoriasis patients receiving PUVA, UVB, coal tar, radiation therapy and other immunosuppressants
• Capsorin is contra-indicated in nephrotic syndrome patients with uncontrolled hypertension, uncontrolled infections or any kind of malignancy
• Capsorin should not be used to treat rheumatoid arthritis in patients under the age of 18 years.
• Concomitant use of tacrolimus is specifically contraindicated.
• Concomitant use of Hypericum perforatum (St. John’s Wort) drastically reduces the plasma concentration of ciclosporin. This may result in a loss of therapeutic effect (see section 4.5)
• Concomitant use of rosuvastatin is specifically contraindicated.
Capsorin should be prescribed only by physicians specialising in organ transplantation, dermatology, nephrology or rheumatology. Patients should be monitored in facilities with sufficient laboratory capacity and supporting medical resources. The responsible physician should have all the available information in preparation for the follow-up patients.
Capsorin should not be given in combination with other calcineurin inhibitors such as tacrolimus, since this can be expected to lead to an increase in adverse effects (see also 4.5 Interactions with other medicinal products and other forms of interactions) without an improvement in efficacy.
In patients being given Capsorin, the use of potassium-sparing diuretics, medicinal products containing potassium, ACE inhibitors, angiotensin-II-receptor antagonists and a high intake of potassium with food should be avoided
Grapefruit juice may elevate the blood levels of ciclosporin by interacting with the cytochrome-P450 system. The extent of these changes of ciclosporin levels in the blood, however, differs in individual cases and is not predictable. Therefore, grapefruit juice should not be taken in conjunction with Capsorin.
The use of medicinal products that can cause gingival hyperplasia (e. g. nifedipine) should be avoided in patients who develop gingival proliferation under Capsorin (see “4.8 Undesirable effects”).
When using inactivated vaccines or toxoid vaccines, the immune response should always be controlled by means of titer determination (see “4.5 Interaction with other medicinal products and other forms of interaction
Caution should be exercised in patients with hyperuricaemia since ciclosporin may further elevate uric acid levels.
Capsorin may impair renal function. For this reason, a reliable creatinine baseline value must be established prior to therapy with Capsorin. In the first three months of treatment, the serum creatinine and serum urea values must be checked every two weeks.
In the event that kidney transplant patients who have very high ciclosporin levels in the blood present with continuously worsening renal function values and if the latter do not respond to a corresponding dose reduction, more extensive diagnostic tests should be conducted, e. g. a kidney biopsy.
Capsorin may also impair liver function. For this reason the parameters for liver function should be checked on a routine basis.
Since ciclosporin may on occasion precipitate hyperkalaemia or hypomagnesaemia or exacerbate existing electrolytic disturbances of this kind, it is recommended serum potassium and magnesium levels be monitored, particularly in patients with marked renal dysfunction.
During treatment with ciclosporin, a routine blood pressure check is required (see “4.8. Undesirable effects”). Treatment with ciclosporin should be discontinued is hypertension cannot be controlled with appropriate hypertensive treatment.
When taking cyclosporine, a reversible elevation of blood lipids may occur. For this reason it is recommended that blood lipid values be determined prior initiating treatment and following the first month of treatment. Should blood lipids become elevated, the intake of fats with food should be restricted and/or the cyclosporine dose should be reduced.
Routine dental check-ups (e. g. every three months) are recommended. In order to preclude or reduce gingival hyperplasia, teeth should be cleaned professionally and the patient should be instructed about measures necessary for personal dental hygiene.
Under ciclosporin treatment, there is an increased frequency of skin tumours. For this reason, patients should be warned against unnecessary radiation from the sun. A routine examination of the skin as well as histological examination of suspicious alterations is recommended.
Particular caution is advised in patients with untreated acute infections.
The routine determination of the minimum ciclosporin concentration in whole blood is an important safety measure within the scope of therapy monitoring in transplant patients (see “4.2 Posology and method of administration” under “Organ transplantation”).
It should be taken into account that the determination of the ciclosporin levels in whole blood, plasma, or serum is only one of the factors contributing to the clinical assessment of the patient’s status. Therefore, blood cyclosporine levels should only serve as a reference for treatment and are to be supplemented by additional clinical and laboratory parameters.
Capsorin may increase the risk of benign intracranial hypertension. Patients presenting with signs of raised intracranial pressure should be investigated and if benign intracranial hypertension is diagnosed, ciclosporin should be withdrawn due to the possible risk of permanent visual loss.
Further precautions in nephrotic syndrome
Since ciclosporin may reduce the renal function, frequent monitoring is necessary and if the serum creatinine levels are more than 30% above baseline in more than one measurement, the dose of ciclosporin should be reduced by 25-50%. Patients with abnormal baseline for renal function should be treated initially with 2.5 mg/kg/day and monitored carefully.
It should be noted that in some nephrotic syndrome itself can cause alterations in renal function. Thus, structural kidney alterations have been observed in association with ciclosporin treatment, without an increase in serum creatinine levels. Renal biopsy is indicated in patients treated with ciclosporin for more than one year to access the progression of renal disease and the extent of any ciclosporin-associated changes in renal morphology that may co-exist.
In patients with nephrotic syndrome treated with immunosuppressants (including ciclosporin), there have been reports of malignant growths (including Hodgkin's lymphoma).
Long-term data on ciclosporin in the treatment of nephrotic syndrome are limited. However, in clinical trials patients have received treatment for 1 to 2 years. Longterm treatment may be considered if there has been a significant reduction in proteinuria with preservation of creatinine clearance and provided adequate precautions are taken.
Further precautions in rheumatoid arthritis
Since ciclosporin may reduce renal function, a reliable baseline for serum creatinine in at least to measurements should be established before treatment. Afterwards, serum creatinine levels should be monitored weekly for one month. Thereafter serum creatinine should be monitored every two weeks in the first 3 months of treatment and thereafter once a month. More frequent control is required when the ciclosporin dose is increased, if concomitant treatment with a non-steroidal anti-inflammatory substance is initiated or increased.
If serum creatinine levels are more than 30% above baseline in several measurements, the dose of Capsorin should be reduced. If serum creatinine levels increase by more than 50%, the dose should be reduced by 50%. These recommendations apply even if values are within the laboratory normal range. If serum creatinine levels do not decrease within one month, treatment with Capsorin should be discontinued.
Treatment should also be discontinued if treatment-emergent hypertension cannot be controlled with appropriate antihypertensive treatment.
As with other long immunosuppressive treatments there is an increased risk of lymphoproliferative disturbances. Caution is advised if ciclosporin is used concomitantly with methotrexate.
When treating rheumatoid arthritis, and taking into consideration the safety of the patient, additional controls should be carried out in accordance with the following time frame:
- haematology profile (red blood count, leucocyte and thrombocyte counts): primary and thereafter every 4 weeks
- liver enzymes: primary and thereafter every 4 weeks
- urine status: primary and thereafter every 4 weeks
- blood pressure: primary and thereafter every 2 weeks for 3 months. Afterwards, every 4 weeks.
- potassium, lipids: primary and thereafter every 4 weeks.
Experience is available from clinical studies for a period of up to 12 months. There is currently insufficient experience for longer treatment periods. If there is no perceptible effect after 3 months of treatment, administration with Capsorin should be discontinued.
Since ciclosporin may reduce the renal function, a reliable baseline for serum creatinine in at least two measurements should be established before the treatment, and serum creatinine should be monitored every 2 weeks in the first 3 months of the treatment and thereafter once a month. If serum creatinine levels increases to more than 30% above baseline and are continually increased in more than one measurement, Capsorin dose should be reduced by 25 to 50%. If serum creatinine level increases by more than 50%, the dose should be reduced by 50%. These recommendations apply even if values are within the normal laboratory range. If serum creatinine levels do not decrease within one month, treatment with Capsorin should be discontinued.
The treatment should also be discontinued if treatment emergent hypertension cannot be controlled with appropriate antihypertensive treatment.
Elderly patients should only be treated if their psoriasis is debilitating and their renal function should be monitored carefully.
The duration of use is normally 12 weeks. Insufficient experience exists with treatment regimens longer than 24 weeks in duration. Termination of the treatment is recommended if high blood pressure which cannot be adequately controlled occurs during treatment with Capsorin.
Development of malignant growths (especially of the skin) has been reported in psoriasis patients receiving treatment with ciclosporin as well as those treated with traditional immunosuppressants. A scan for all forms of pre-existing tumours, including those of the skin and cervix, should be carried out. A biopsy should be performed before starting Capsorin treatment on skin lesions which are not typical for psoriasis to exclude skin cancers, mycosis fungoides or other premalignant disorders. Patients with malignant or premalignant skin alterations should only be treated with ciclosporin after appropriate treatment of these lesions and only if there is no alternative treatment.
A small number of psoriasis patients on ciclosporin treatment have developed lympho-proliferative disturbances which were reversible by immediate discontinuation of treatment. Patients treated with Capsorin should not receive concomitant irradiation treatment with UV-B-radiation or PUVA-photochemotherapy.
In view of the potential risk of skin malignancy, patients on Capsorin should be warned to avoid excessive unprotected sun exposure.
Further precautions in atopic dermatitis
Since ciclosporin may reduce the renal function, a reliable baseline for serum creatinine in at least two measurements should be established before the treatment, and serum creatinine should be monitored every 2 weeks in the first 3 months of the
treatment and thereafter once a month. If serum creatinine levels increase to more than 30% above baseline and are continuously increased in more than one measurement, the dose of Capsorin should be reduced by 25-50%. These recommendations apply even if values are within the normal laboratory range. If serum creatinine levels do not decrease within one month treatment with ciclosporin should be discontinued.
Treatment should also be discontinued if treatment emergent hypertension cannot be controlled with appropriate antihypertensive treatment.
Since experience with ciclosporin in children with atopic dermatitis is limited, Capsorin is not recommended for use in children.
Elderly patients should only be treated if their atopic dermatitis is debilitating, and their renal function should be monitored carefully.
Benign lymphadenopathy is often connected with flare-up of atopic dermatitis and disappears spontaneously or with improvement in the disease. Lymphadenopathy observed in association with ciclosporin treatment should be monitored carefully. If lymphadenopathy continues despite improvement, a preventive biopsy should be made to exclude the possibility of lymphoma.
Active Herpes simplex infections should be eliminated before treatment with ciclosporin is initiated, but discontinuation of ciclosporin treatment is only warranted if severe infection develops during treatment.
Skin infections with Staphylococcus aureus are not an absolute contra-indication for treatment with Capsorin, but should be treated with appropriate antibacterial drugs. Oral erythromycin may increase the blood concentration of ciclosporin (see section 4.5. Interaction with other medicinal products and other forms of interaction) and should therefore be avoided. If there is no alternative available, blood concentrations of ciclosporin, renal function and possible adverse reactions to ciclosporin should be monitored closely.
Since there is a potential risk of malignant skin growths, patients treated with ciclosporin should be cautioned against exposure to sunlight without protection. These patients should not receive concomitant radiotherapy with UVB radiation or PUVA photochemotherapy.
Capsorin contains 12.7 vol% ethanol (alcohol), i.e. up to 525mg per dose, equivalent to 13ml beer, 6ml wine per dose. Harmful for those suffering from alcoholism. To be taken into account in pregnant or breast-feeding woman, children and high-risk groups such as patients with liver disease, or epilepsy.
4.5 Interaction with other medicinal products and other forms of interaction Interaction with foods
Concomitant administration of grapefruit juice has been shown to increase the bioavailability of ciclosporin.
Interaction with other medicines
The section below list the medicines for which an interaction with Ciclosporin has been sufficiently proven and is considered to be clinically relevant.
Different medicines either increase or decrease the plasma or whole blood concentration of ciclosporin, usually by inhibition or induction of enzymes involved in the metabolism of ciclosporin (particularly cytochrome P450)
The product contains ethanol (see section 4.4). Ethanol may interact with other medicinal products.
Medicines which reduce ciclosporin concentrations:
Barbiturates, carbamazepine, phenytoin; phenobarbital; primidone; griseofulvin; metamizole; nafcillin, sulfadimidine and trimethoprim i.v.; rifampicin; octreotide; probucol; sulphadiazine, orlistat, troglitazone, Hypericum perforatum (St John's Wort), ticlopidine.
Patients on ciclosporin treatment should not use products/herbal medicines, which contain Hypericum perforatum, since this may cause a marked reduction in plasma concentrations of ciclosporin by induction of CYP3A4, and thus a diminution of therapeutic efficacy (see 4.3 Contraindications).
Medicines which increase ciclosporin concentrations:
Macrolide antibiotics (mainly erythromycin, clarithromycin, josamycin, roxithromycin and pristinamycin); ketoconazole, fluconazole, itraconazole; calcium antagonists (such as diltiazem, nicardipine, verapamil); metoclopramide; oral contraceptives; propafenone; danazol; methylprednisolone (high dose); allopurinol; anti-H2 (cimetidine, ranitidine); chloroquine; amiodarone; bromocriptine; protease inhibitors, doxycycline..
Other relevant forms of interaction with other medicines
Caution is advised when the concomitant use of other medicines with ciclosporin results in nephrotoxic synergy: aminoglycosides (including gentamicin, tobramycin), amphotericin B, ciprofloxacin, vancomycin, trimethoprim (+ sulfamethoxazole); nonsteroidal anti-inflammatory substances (including diclofenac, naproxen, sulindac); melphalan, tacrolimus and sirolimus.
During treatment with ciclosporin vaccinations may be less effective, so the use of live weakened vaccine should be avoided.
Concomitant administration of nifedipine and ciclosporin may exacerbate the gingival hyperplasia that is seen when ciclosporin is used alone.
When combining ciclopsporin with corticosteroids, methylprednisolone, prednisone, or prednisolone, an increased risk of brain seizures has been reported. This is especially true for high doses of corticosteroids.
Concomitant use of diclofenac and ciclosporin has shown to cause a pronounced increase in the bioavailability of diclofenac, which may cause reversible reduced renal function. The increase in the bioavailability of diclofenac is most probably due to a reduction in the high first-pass effect of diclofenac. If non-steroidal antiinflammatory substances with a low first-pass effect (e.g. acetylsalicylic acid) are used concomitantly with ciclosporin, no increase in bioavailability is expected.
Ciclosporin may also reduce the excretion of digoxin, colchicine, lovastatin, pravastatin, simvastatin, atorvastatin and prednisolone and may therefore lead to digoxin toxicity or increase the risk of muscle toxicity (including muscle pain and weakening, myositis and occasionally rhabdomyolysis) due to colchicin, lovastatin, pravastatin, simvastatin and atorvastatin.
Recommendations
If concomitant use of medicines which have an interaction with ciclosporin in unavoidable, the following basic recommendations should be followed:
During concomitant use of medicines which cause nephrotoxic synergy, renal function (especially serum creatinine) should be monitored carefully. If renal function is considerably reduced, the dose of the concomitant medicine should be decreased or an alternative treatment should be considered.
Medicines which are known to reduce or increase the bioavailability of ciclosporin:
In transplant patients frequent measurement of ciclosporin concentrations is required with possible dose adjustment, especially during initiation of treatment or at discontinuation of the concomitant medicine. In non-transplant patients, the value of monitoring blood concentrations of ciclosporin is doubtful, since the relationship between blood concentration and clinical effect is not well established. If medicines which increase the ciclosporin concentrations are used concomitantly, it may be more useful to measure renal function frequently and to monitor the patient carefully with regards to ciclosporin related adverse reactions.
Concomitant use of nifedipine should be avoided in patients with gingival hyperplasia.
Non-steroidal anti-inflammatory substances which are known to have a marked first-pass metabolism (e.g. diclofenac) should be given at a lower dose than that normally recommended for patients not receiving ciclosporin.
As hepatotoxicity is a potential side effect of non-steroidal anti-inflammatory drugs, regular monitoring of hepatic function is advised when Ciclosporin is co-administered with these drugs in rheumatoid arthritis patients.
If digoxin, colchicine, lovastatin, pravastatin or simvastatin are used concomitantly with ciclosporin, carefully clinical monitoring is required.
4.6 Pregnancy and lactation
Pregnancy:
Experience with ciclosporin in pregnant women is limited.
Ciclosporin does not demonstrate teratogenicity in experimental animals. Limited experience regarding the safety of administration of ciclosporin to pregnant women has shown no indications of teratogenicity. Ciclosporin does pass into the placenta. Initial experience with transplantation patients, however, did indicate that ciclosporin, as with other immunosuppressive agents, increases the probability of specific complications during pregnancy, such as pre-eclampsia and premature births with decreased birth weights.
Capsorin should be given during pregnancy only when the benefits outweigh the risks. Pregnant women who are being treated with Capsorin should be observed carefully.
Lactation:
Ciclosporin is excreted in the breast milk. Women receiving Capsorin treatment should not breast feed.
4.7 Effects on ability to drive and use machines
No data exist on the effects of ciclosporin on ability to drive and use machines.
The product contains ethanol (see section 4.4). Ethanol may have an influence on the ability to drive and use machines.
4.8 Undesirable effects
Many of the adverse reactions to ciclosporin are dose dependent and can be avoided by dose reduction. The adverse reactions are generally the same in the different indications, but occur at different frequencies. Since a higher initial dose and longer maintenance treatment is required after transplantation, adverse reactions are seen more frequently and are usually more severe in transplant patients than in patients treated for other indications.
Frequency estimates:
Very common (>1/10)
Common (>1/100 to <1/10)
Uncommon (>1/1,000 to <1/100)
Rare (>1/10,000 to <1/1,000)
Very rare (<1/10,000), not known (cannot be estimated from the available data)
Blood and lymphatic system disorders
Uncommon: Anaemia, thrombocytopaenia.
Rare: Microangiopathic haemolytic anaemia, haemolytic uraemic syndrome. Endocrine disorders
Uncommon: In some patients malignant neoplasia or lymphoproliferative diseases have been reported, with incidence and distribution similar to those in patients receiving traditional immunosuppressive therapy.
Rare: Menstrual disturbances, gynecomastia.
Metabolism and nutrition disorders
Very common: Hyperlipidaemia, hypercholesterolaemia.
Common: Hyperuricaemia, hyperkalaemia, hypomagnesaemia.
Rare: Hyperglycaemia.
Nervous system disorders
Very common: Tremor, headache.
Common: Paresthesia.
Uncommon: Signs of encephalopathy, e.g. convulsion, confusion, disorientation, decreased responsiveness, agitation, insomnia, visual disturbances, cortical blindness, coma, paresis, cerebellar ataxia.
Rare: Motor polyneuropathy.
Very rare: Oedema in the visual pupil, including disk oedema with possible visual weakening following benign intracranial hypertension.
Vascular disorders
Very common: hypertension
Gastrointestinal disorders
Common: Anorexia, nausea, vomiting, abdominal pain, diarrhoea, gingival hyperplasia.
Hepatobiliary disorders
Common: Hepatic dysfunction.
Rare: Pancreatitis.
Skin and subcutaneous tissue disorders
Common: Hypertrichosis.
Uncommon: Allergic rash.
Musculoskeletal and connective tissue disorders
Common: Muscle cramps, myalgia.
Rare: Muscle weakness, myopathy.
Renal and urinary disorders
Very common: Renal dysfunction (see 4.4 Special warnings and precautions for use).
General disorders and administration site conditions
Common: Fatigue.
Uncommon: Oedema, weight gain.
4.9
Overdose
a) Symptoms of intoxication
Little experience exists with overdose.
After ingestion of doses up to 10 g ciclosporin (approximately 150 mg/kg), vomiting, somnolence, headache, tachycardia and in some patients a moderately severe, reversible kidney dysfunction was observed. There have been reports of severe intoxication symptoms with preterm infants after inadvertent parenteral overdose.
b) Therapy of intoxication
Possible signs of nephrotoxicity are reversible in most cases after discontinuation of administration of Capsorin. In case of an overdose, symptomatic treatment and general supportive measures should be applied. Ciclosporin is not dialyzable nor is it eliminated by activated charcoal-haemoperfusion therapy.
Therefore, elimination is limited to non-specific treatment, e. g. gastrolavage. However, activated charcoal eliminates small amounts of ciclosporin from the enterohepatic circulation. Within the first few hours after overdose, it may be beneficial for the patient to induce vomiting.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Immunosuppressants ATC code: L04A D01.
Ciclosporin (also called cyclosporine A) is a cyclic poly peptide, which consists of 11 amino acids. It is a strong immunosuppressive substance, which in animals increases the survival of allogenic transplantations of skin, heart, kidneys, pancreas, bone marrow, small intestine and lungs. Studies show that ciclosporin inhibits the development of cell-mediated reactions, including allotransplantation immunity, delayed skin hypersensitivity, experimental allergic encephalomyelitis, Freund’s adjuvant arthritis, graft-versus-host disease (GVHD) and the production of T-cell dependent antibodies. At the cellular level ciclosporin inhibits the production and release of lymphokines, including interleukin 2 (T-cell growth factor, TCGF). Ciclosporin apparently blocks the resting lymphocytes in phase G0 or G1 in the cell cycle and inhibits the antigen triggered release of lymphokines from activated T-cells.
The existing evidence indicates that ciclosporin acts specifically and reversibly on lymphocytes. Contrary to cytostatic agents, ciclosporin does not suppress haemopoiesis and has no effect on phagocytic cell function. Patients treated with ciclosporin are less susceptible to infections than those treated with other immunosuppressants.
Successful organ and bone marrow transplantations have been carried out in humans, where ciclosporin has been used to prevent and treat rejection and GVHD.
Treatment with ciclosporin has also shown to be advantageous in a series of other conditions with a known autoimmune origin or considered to be of autoimmune origin.
5.2 Pharmacokinetic properties
The maximal blood concentration (Cmax) is achieved within 1-2 hours (Tmax). The absolute bioavailability is 30-60%. The inter- and intra-individual pharmacokinetic variability is 10-20% for AUC and Cmax in healthy volunteers. Capsorin can be administered with food or alone.
The results of several studies have shown that monitoring of the ciclosporin area under the time-concentration curve for the first 4 hours after administration of dose (AUC0-4) gives a more precise prediction of the ciclosporin exposure than at base (C0) monitoring.
The results from further studies indicate that a single test point 2 hours after the dose (C2) correlate well with the AUC0-4 in transplantation patients.
In medical practice either trough level monitoring or C2 monitoring of ciclosporin can be used for pharmacotherapeutic surveillance.
Ciclosporin is mainly distributed outside the blood volume. In the blood there is 3347% ciclosporin in plasma, 4-9% in the lymphocytes, 5-12% in the granulocytes and 41-58% in the erythrocytes. In plasma approximately 90% is bound to proteins, mainly lipoproteins.
Ciclosporin is biotransformed by several metabolic routes into approximately 15 metabolites. The elimination is mainly biliary, where only 6% of an oral dose is eliminated with the urine. Only 0.1% is eliminated unchanged in the urine.
There is a great variation in the available data regarding the terminal half life of ciclosporin depending on the analysis and the target population. The terminal half life varied from 6.3 hours in healthy volunteers to 20.4 hours in patients with severe hepatic disease.
5.3 Preclinical safety data
Ciclosporin showed no mutagenic or teratogenic effects in appropriate test systems.
Reproduction studies in rats showed only negative effects at doses, which were toxic for the females. At toxic doses (rats 30mg/kg and rabbits 100mg/kg/day orally), ciclosporin was embryo- and foeto-toxic, which was indicated by increased prenatal and postnatal mortality and reduced foetal weight and bone formation.
Within the well-tolerated dose range (in rats up to 17 mg/kg/day and in rabbits up to 30mg/kg/day orally), ciclosporin showed no embryo-lethal or teratogenic effects.
Carcinogenicity studies were carried out in male and female rats and mice. In the mouse study, which lasted 78 weeks, there was a statistically significantly greater incidence of lymphocytic lymphomas in female mice at a dose of 1, 4, and 16mg/kg/day and a considerably higher occurrence of hepatocellular carcinomas in male mice, compared to control animals. In the rat study, which lasted 24 months and involved a dose of 0.5, 2 and 8mg/kg/day, the incidence of island cell adenomas in the pancreas considerably exceeded the control value at the low dose. Hepatocellular carcinomas and island cell adenomas in the pancreas were not dose related.
Studies in male and female rats showed no reduction in fertility.
Ciclosporin was not found to be mutagenic/genotoxic in the Ames test, the V79-HGPRT test or the micronucleus test in mice and Chinese hamsters or the chromosome aberration test of the bone marrow of Chinese hamster, the dominating mortality analysis in mice and the DNA repair test in semen from treated mice. An in vitro analysis of sister chromatid exchange (SCE) in human lymphocytes showed a positive effect of ciclosporin at high doses in this system.
An increased occurrence of malignant growths is a recognised complication in connection with immunosuppression in organ transplant patients. The most common forms of neoplasms are non-Hodgkin's related lymphomas and skin carcinomas. The risk of malignant growths during treatment with ciclosporin is higher than in a normal healthy population, but is similar to the risk for patients treated with other immunosuppressants. Reports that reduction or discontinuation of immunosuppressants may cause regression of lesions are available.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Ethanol, anhydrous
Tocopherol acetate Diethylene glycol monoethyl ether Oleoyl macrogolglycerides Macrogolglycerol hydroxystearate
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
3 years
The oral solution should be used within 2 months after first opening.
6.4 Special precautions for storage
Do not store above 25°C.
6.5 Nature and contents of container
Amber glass bottles type III, available in 50 ml with plastic cover and aluminium cap. Dispenser sets are also provided, one for adult and other for paediatric use.
Capsorin100 mg/ml oral solution is available in pack size of 1 bottle of 50 ml.
6.6 Special precautions for disposal
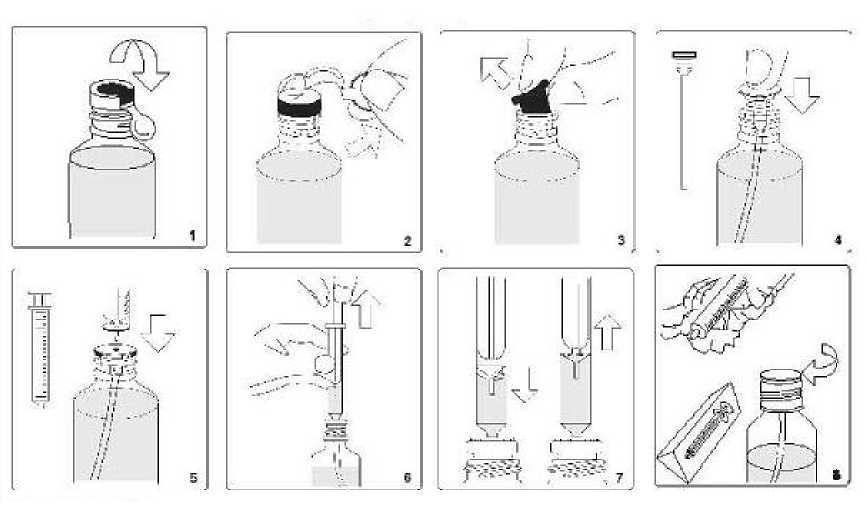
1) Raise flap on top of the metallic sealing ring
2) Tear off sealing ring completely
3) Remove rubber stopper from bottle and dispose of it carefully
4) Insert the tube with attached white stopper into the bottle, and press until the stopper is firmly into the neck of the bottle
5) Insert the nozzle of syringe (doser) into the white stopper
6) Draw up the plunger until the medicine rises up the syringe to the dosage level prescribed by your doctor
7) If any larger bubbles of air appear in the syringe, push the plunger all the way down and aspirate the solution with various times to force the bubbles out. After the large bubbles are gone, measure the prescribed solution volume again. The presence of a few tiny bubbles will not affect the dosing in any way
8) After use, clean the exterior of the syringe with a clean dry tissue and return to its protective cover. The white stopper and tube should remain permanently in the bottle. Close bottle with the screw-top
Next use: The next time you use your medicine, start at Point 5
7 MARKETING AUTHORISATION HOLDER
Morningside Healthcare Ltd
115 Narborough Road
Leicester
LE30PA
UK
8 MARKETING AUTHORISATION NUMBER(S)
PL 20117/0043
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
02/07/2010
10 DATE OF REVISION OF THE TEXT
02/07/2010