Cefuroxime 750 Mg Powder For Solution For Injection
Out of date information, search another
Anexo 6.00 do PT.G.087
|
CODIGO: 796678 Code |
DESIGNA?A°: LIT.CEFUROXIME 0,75G INJ FKUK Name |
ELABORADO POR: , , ^ Made by Jose Duarte |
|
VERSA0: /xx Version |
PROVA: n- Proof Ul* |
E™ 10 Abr.2014 |
|
FICHA TECNICA: 796678 Technical Sheet |
ESCALA: Scale 1:1 |
Text size: 8 ptd |
|
CORES: |
• |
Black |
|
Colours |
• |
Cut |
|
o | ||
|
o | ||
|
o |
Package Leaflet: Information for the user
Cefuroxime 750 mg
powder for solution for injection/infusion
Cefuroxime

Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.

What is in this leaflet:
1. What Cefuroxime is and what it is used for
2. What you need to know before you use Cefuroxime
3. How to use Cefuroxime
4. Possible side effects
5. How to store Cefuroxime
6. Contents of the pack and other information
1. What Cefuroxime is and what it is used for
Cefuroxime is an antibiotic used in adults and children. It works by killing bacteria that cause infections. It belongs to a group of medicines called cephalosporins.
Cefuroxime is used to treat infections of:
• the lungs or chest
• the urinary tract
• the skin and soft tissue
• the abdomen
Cefuroxime is also used:
• to prevent infections during surgery.
You must not be given Cefuroxime:
• if you are allergic (hypersensitive) to any cephalosporin antibiotics or any of the other
ingredients of Cefuroxime.
• if you have ever had a severe allergic (hypersensitive) reaction to any other type of betalactam antibiotic (penicillins, monobactams and carbapenems).
->Tell your doctor before you start on Cefuroxime if you think that this applies to you. You must not be given Cefuroxime.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using Cefuroxime. You must look out for certain symptoms such as allergic reactions and gastrointestinal disorders such as diarrhoea while you are being given Cefuroxime. This will reduce the risk of possible problems. See (‘Conditions you need to look out for1) in section 4. If you have had any allergic reaction to other antibiotics such as penicillin, you may also be allergic to Cefuroxime.
If you need a blood or urine test
Cefuroxime can affect the results of urine or blood tests for sugar and a blood test known as the Coombs test. If you are having tests:
->Tell the person taking the sample that you have been given Cefuroxime.
Other medicines and Cefuroxime
Tell your doctor if you are taking any other medicines, if you've started taking any recently or you start taking new ones. This includes medicines you can obtain without a prescription.
Some medicines may affect how Cefuroxime works, or make it more likely that you'll have side effects. These include:
• aminoglycoside-type antibiotics
• water tablets (diuretic), such as furosemide
• probenecid
• oral anticoagulants
->Tell your doctor if this applies to you. You may need extra check-ups to monitor your renal function while you are taking Cefuroxime.
Contraceptive pills
Cefuroxime may reduce the effectiveness of the contraceptive pill. If you are taking the contraceptive pill while you are being treated with Cefuroxime you also need to use a barrier method of contraception (such as condoms). Ask your doctor for advice.
Pregnancy and breast-feeding and fertility
Tell your doctor before you are given Cefuroxime:
• if you are pregnant, think you might be pregnant or are planning to become pregnant
• if you are breastfeeding
Your doctor will consider the benefit of treating you with Cefuroxime against the risk to your baby.
Driving and using machines
Don't drive or use machines if you do not feel well.
Important information about some of the ingredients of Cefuroxime
Cefuroxime contains sodium. You need to take this into account if you are on a controlled sodium diet.
|
Cefuroxime strength |
Amount per vial |
|
750 mg |
40.63 mg |
3. How to use Cefuroxime
Cefuroxime is usually given by a doctor or nurse. It can be given as a drip (intravenous infusion) or as an injection directly into a vein or into a muscle.
The usual dose
The correct dose of Cefuroxime for you will be decided by your doctor and depends on: the severity and type of infection, whether you are on any other antibiotics; your weight and age; how well your kidneys are working.
Newborn babies (0 - 3 weeks)
For every 1 kg the baby weighs, they’ll be given 30 to 100 mg Cefuroxime per day divided in two or three doses.
Babies (over 3 weeks) and children
For every 1 kg the baby or child weighs, they’ll be
given 30 to 100 mg of Cefuroxime per day divided in three or four doses.
Adults and adolescents
750 mg to 1.5 g of Cefuroxime per day divided into two, three or four doses. Maximum dose: 6 g per day.
Patients with kidney problems
If you have a kidney problem, your doctor may change your dose.
->Talk to your doctor if this applies to you.
4. Possible side effects
Like all medicines this medicine can cause side effects, although not everybody gets them.
Conditions you need to look out for
A small number of people taking Cefuroxime get an allergic reaction or potentially serious skin reaction. Symptoms of these reactions include:
• severe allergic reaction. Signs include raised and itchy rash, swelling, sometimes of the face or mouth causing difficulty in breathing.
• skin rash, which may blister, and looks like small targets (central dark spot surrounded by a paler area, with a dark ring around the edge).
• a widespread rash with blisters and peeling skin. (These may be signs of Stevens-Johnson syndrome or toxic epidermal necrolysis).
• fungal infections on rare occasions, medicines like Cefuroxime can cause an overgrowth of yeast (Candida) in the body which can lead to fungal infections (such as thrush). This side effect is more likely if you take Cefuroxime for a long time.
-> Contact a doctor, pharmacist or nurse immediately if you get any of these symptoms.
Common side effects
These may affect up to 1 in 10 people:
• injection site pain, swelling and redness along a vein. ->Tell your doctor if any of these are troubling you.
Common side effects that may show up in blood tests:
• increases in substances (enzymes) produced by the liver
TJ -c
CJ Q_
IZ
CL) n3
Q-T3
1/1 QJ
?! E
ftJ Q_
<
E ^ "2
CD i/i
o t;
c= m >
2 -o
.E S o S .§■2 #1 o 1/1
_Q CD
QJ
O CD c _c: >
lo o o
M— LO
o r~- E
o *=
<= -Q3
O 1/1 ' ro ^ 0-13 o
3 E
i_ -o
^ CD
> 1/1 4_l
Q. O CD cn -a
qj a .G CD
S
P _a T3 q) CD « JD §
o ElB
CD -Q C_i +3
-Cl * CD -G ^
I I § |.s Util
Id ~3 c; ^ a | sJ I G
^ ‘C Si c~
8 -S tS s fi ■8 ?!■§
Q.
.§
3 G P
c; CD \ 5) Q 6,
fit
!f.s
r
ll I
This medicinal product is authorised in the Member States of the EEA under the following names:
|
Name of the Member State |
Name of the medicinal product |
|
Belgium |
Cefuroxim Fresenius Kabi 750 mg, poeder voor oplossing voor injectie of infusie, poudre pour solution injectable ou pour perfusion, Pulver zue Herstellung einer Injektionslosung/lnfusionslosung |
|
Czech Republic |
Cefuroxim Kabi 750 mg |
|
Denmark |
Cefuroxim Fresenius Kabi 750 mg |
|
Germany |
Cefuroxim Kabi 750 mg Pulver zur Herstellung einer Injecktions-/ Infusionslosung |
|
Greece |
CefuroximeKabi xovig yia Evcaipo bidAupa, 750 mg |
|
France |
CEFUROXIME KABI 750 mg, poudre pour solution injectable ou pour perfusion |
|
Hungary |
Cefuroxim Kabi 750 mg por oldatos injekciohoz vagy infuziohoz |
|
Ireland |
Cefuroxime 750 mg powder for solution for injection/infusion |
|
Netherlands |
Cefuroxim Fresenius Kabi 750 mg, poeder voor oplossing voor injectie /infusie |
|
Norway |
Cefuroxim Fresenius Kabi 750 mg |
|
Poland |
Cefuroxim Kabi |
|
Sweden |
Cefuroxim Fresenius Kabi 750 mg pulver till injektions-Musionsvatska, losning |
|
Slovak Republic |
Cefuroxim Kabi 750 mg |
|
United Kingdom |
Cefuroxime 750 mg powder for solution for injection/infusion |
This leaflet was last revised in Mar 2014
• changes in your white blood cell count (neutropaenia or eosinophilia)
• low levels of red blood cells (anaemia)
Uncommon side effects
These may affect up to 1 in 100 people:
• skin rash, itchy, bumpy rash (hives)
• diarrhoea, nausea, stomach pain
-> Tell your doctor if you get any of these.
Uncommon side effects that may show up in blood tests:
• low levels of white blood cells (ieucopaenia)
• increase in bilirubin (a substance produced by the liver)
• positive Coomb's test.
Other side effects
Other side effects have occurred in a very small number
of people but their exact frequency is unknown:
• fungal infections
• high temperature (fever)
• allergic reactions
• inflammation of the colon (large intestine), causing diarrhoea, usually with blood and mucus, stomach pain
• inflammation in the kidney and blood vessels
• red blood cells destroyed too quickly (haemolytic anaemia).
• skin rash, which may blister, and looks like small targets (central dark spot surrounded by a paler area, with a dark ring around the edge) erythema multiformae.
-> Tell your doctor if you get any of these.
Side effects that may show up in blood tests:
• decrease in number of blood platelets (cells that help blood to clot - thrombocytopaenia)
• increase in levels of urea nitrogen and serum creatinine in the blood.
If you get any side effects
->Tell your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. By reporting side effects you can help provide more information on the safety of this medicine. For UK - You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard For Ireland - Reports may be made by following the links to the online reporting option accessible from the IMB homepage, or by completing the downloadable report form also accessible from the IMB website, which may be completed manually and submitted to the IMB via freepost, to the following address:
FREEPOST
IMB Pharmacovigilance Earlsfort Terrace
IRL-Dublin 2Tel: +353 1 6764971 Fax: +353 1 6762517 Website: www.imb.ie(http://www.imb.ie) e-mail: imbpharmacovigilance@imb.ie
5. How to store Cefuroxime
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the pack after EXP. The expiry date refers to the last day of that month.
Do not store above 25°C. Keep the vials in outer carton in order to protect from light
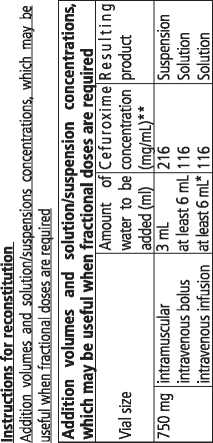
Once Cefuroxime powder is made up into a suspension/ solution for injection, it should be used immediately. If not used immediately, the ready-to-use solution/ suspension should be stored in the refrigerator (between 2 - 8 °C) and used within 5 hours.
Do not use Cefuroxime if you notice visible signs of deterioration such as particulate matter and discoloration. Any unused solution/suspension should be thrown away.
Do not throw away any medicines via wastewater or household waste. Your doctor or nurse will dispose of
any medicine that is no longer required. These measures will help to protect the environment.
6. Contents of the pack and other information
What Cefuroxime contains:
The active substance is cefuroxime (750 mg) as cefuroxime sodium.
What Cefuroxime looks like and contents of the pack:
Cefuroxime powder is normally mixed with water for injection to make up a clear solution for injection or infusion into veins (intravenous) or to make up a suspension for injection into muscles (intramuscular). Once made up, your doctor may mix the Cefuroxime solution with other suitable fluids for infusion. Solutions and suspensions can range in colour from clear to yellow coloured depending on concentration, diluent and storage conditions.
Cefuroxime 750 mg powder for solution for injection/ infusion comes in packs containing 1 and 10 glass vials of powder, closed with rubber stoppers, aluminium caps and blue plastic flip-off caps together.
Not all the pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer:
Marketing Authorisation holder:
Fresenius Kabi Limited Cestrian Court,
Eastgate Way,
Manor Park, Runcorn, Cheshire, WA7 1NT, UK Manufacturer:
Labesfal - LaboratoriosAlmiros SA Lagedo, 3465-157 Sanitago Besteiros, Portugal.
% FRESENIUS I KABI
UK-IE V002 7966787XX