Celluvisc 0.5% W/V Eye Drops Solution
Ref: 0107/230916/1/F
Celluvisc® 0.5% w/v Eye Drops Solution
(carmellose sodium)
Patient Information Leaflet
a
s
a
s
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
This medicine is available without prescription from pharmacies. Always use this medicine exactly as described in this leaflet or as your pharmacist or nurse has told you.
* Keep this leaflet. You may need to read it again.
* Ask your pharmacist if you need more information or advice.
* If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
* You must talk to a doctor if you do not feel better or if you feel worse.
Your medicine is called Celluvisc 0.5% w/v Eye Drops Solution, however thorughout the rest of this leaflet it will be referred to as Celluvisc
What is in this leaflet
What Celluvisc is and what it is used for What you need to know before you use Celluvisc
How to use Celluvisc
Possible side effects
How to store Celluvisc
Contents of the pack and other information
■1 What Celluvisc is and what it is used for
Celluvisc is a substitute for tears, and contains the lubricant called carmellose sodium. It is used for the treatment of the symptoms of dry eye (such as soreness, burning, irritation or dryness) caused by you not producing enough tears to keep the eye wet.
*2 What you need to know before you use Celluvisc
Do not use Celluvisc
* If you are hypersensitive (allergic) to carmellose sodium or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
* If irritation, pain, redness or changes in vision occur or if you feel your condition is getting worse, stop taking this medicine and consult your doctor or pharmacist.
Other medicines and Celluvisc
Please tell your doctor or pharmacist if you are using, have recently used or might use any other medicines, including medicines obtained without a prescription.
If you are using other eye drops, leave at least 15 minutes between putting in the other drops and Celluvisc
Read all the information in this leaflet before using Celluvisc.
If you are unsure about anything, discuss it with your doctor, nurse or pharmacist.
^ How to use Celluvisc
Always use this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse has told you. Check with your doctor, pharmacist or nurse if you are not sure.
The recommended dose is 1 -2 drops of Celluvisc in the affected eye/each affected eye, 4 times a day or as often as needed.
You do not need to remove contact lenses before using Celluvisc.
Make sure that the single-dose container is intact before use. The solution should be used immediately after opening. To avoid contamination or possible eye injury, do not let the open-end of the single-dose container touch your eye or anything else. Wash your hands before use.
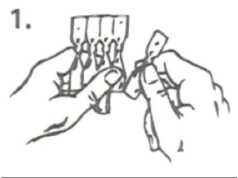

1. Tear one single-dose container from the strip.
2. Hold the single-dose container upright (with the top uppermost) and twist off the top.
Pregnancy and breast-feeding
You can use Celluvisc if you are pregnant and when you are breast-feeding.
Driving and using machines
Celluvisc may cause temporary blurring of vision. If you do experience temporary blurring, do not drive or use machines until your sight is clear.
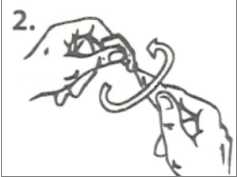
3. Gently pull down the lower eyelid to form a pocket. Turn the single-dose container upside down and squeeze it to release one drop into each eye.
Blink your eyes a few times.
Do not re-use the single-dose container even if there is some solution left. It is most important that you throw it away and do not keep it.
If you use more Celluvisc than you should
It will not cause you any harm. If you are worried, talk to your doctor or pharmacist.
If you forget to use Celluvisc
Use a single drop in each eye that needs treatment as soon as you remember, and then go back to your regular routine. Do not use a large number of drops to make up for forgetting to put drops in your eye or eyes earlier.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
^ Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The side effects are classified into the following categories, depending on how often they occur:
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
^ How to store Celluvisc
Keep out of the sight and reach of children.
Do not store above 25oC
Do not use Celluvisc after the expiry date which is stated on the single-dose container tab and the carton after EXP:. The expiry date refers to the last day of that month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
|
Very common |
may affect more than 1 in 10 people |
|
Common |
may affect up to 1 in 10 people |
|
Uncommon |
may affect up to 1 in 100 people |
|
Rare |
may affect up to 1 in 1,000 people |
|
Very rare |
may affect up to 1 in 10,000 people |
|
Not known |
frequency cannot be estimated from the available data |
The following side effects may be seen with Celluvisc.
|
Common |
Eye irritation (including burning and discomfort), eye pain, itchy eyes, visual disturbance. |
|
Not known |
Allergic reactions (including eye allergy), blurred vision, sticky eye, watery eye, redness of the eye, eye injury to the surface of the eye due to the tip of the vial touching the eye during use. |
" Contents of the pack and other information
What Celluvisc contains
The active ingredient is called carmellose sodium 5mg/ml.
The other ingredients are sodium chloride, sodium lactate, potassium chloride, calcium chloride, magnesium chloride and purified water.
The ingredients and the amount used in Celluvisc were chosen to match what appears in tears produced naturally by the eye.
What Celluvisc looks like and contents of the pack
Celluvisc is a clear colourless eye drop solution, in a single-dose container. The single-dose container has a twist off top.
Each single-dose container contains 0.4ml of solution.
Each pack contains 30 single-dose containers.
Manufacturer and Licence Holder
This medicine is manufactured by Allergan Pharmaceuticals Ireland, Westport, County Mayo, Ireland and is procured from within the EU. Product Licence Holder LTT Pharma Limited, Unit 18, Oxleasow Road, East Moons Moat, Redditch, Worcestershire, B98 0RE. Repackaged by Lexon (UK) Limited, B98 0RE, UK.
If you have any questions or are not sure about anything, ask your doctor or pharmacist. They will have additional information about this medicine and will be able to advise you.
P PL 33723/0107
Revision date: 23/09/16
Celluvisc is a registered tradmark of Allergan, Inc.
Blind or partially sighted?
Is this leaflet hard to see or read? Phone Lexon (UK) Limited, Tel: 01527 505414 for help.
Carmellose sodium
0.5% w/v Eye Drops Solution
Patient Information Leaflet
s
E
E
E
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
This medicine is available without prescription from pharmacies. Always use this medicine exactly as described in this leaflet or as your pharmacist or nurse has told you.
* Keep this leaflet. You may need to read it again.
* Ask your pharmacist if you need more information or advice.
* If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
* You must talk to a doctor if you do not feel better or if you feel worse.
Your medicine is called Carmellose sodium 0.5% w/v Eye Drops Solution, however thorughout the rest of this leaflet it will be referred to as Carmellose sodium
What is in this leaflet
What Carmellose sodium is and what it is used for
What you need to know before you use
Carmellose sodium
How to use Carmellose sodium
Possible side effects
How to store Carmellose sodium
Contents of the pack and other information
M What Carmellose sodium is and what it is used for
Carmellose sodium is a substitute for tears, and contains the lubricant called carmellose sodium. It is used for the treatment of the symptoms of dry eye (such as soreness, burning, irritation or dryness) caused by you not producing enough tears to keep the eye wet.
^ What you need to know before you use Carmellose sodium
Do not use Carmellose sodium
* If you are hypersensitive (allergic) to carmellose sodium or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
* If irritation, pain, redness or changes in vision occur or if you feel your condition is getting worse, stop taking this medicine and consult your doctor or pharmacist.
Other medicines and Carmellose sodium
Please tell your doctor or pharmacist if you are using, have recently used or might use any other medicines, including medicines obtained without a prescription.
If you are using other eye drops, leave at least 15 minutes between putting in the other drops and Carmellose sodium
Pregnancy and breast-feeding
You can use Carmellose sodium if you are pregnant and when you are breast-feeding.
Driving and using machines
Carmellose sodium may cause temporary blurring of vision. If you do experience temporary blurring, do not drive or use machines until your sight is clear.
Read all the information in this leaflet before using Carmellose sodium.
If you are unsure about anything, discuss it with your doctor, nurse or pharmacist.
^ How to use Carmellose sodium
Always use this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse has told you. Check with your doctor, pharmacist or nurse if you are not sure.
The recommended dose is 1-2 drops of Carmellose sodium in the affected eye/each affected eye, 4 times a day or as often as needed.
You do not need to remove contact lenses before using Carmellose sodium.
Make sure that the single-dose container is intact before use. The solution should be used immediately after opening. To avoid contamination or possible eye injury, do not let the open-end of the single-dose container touch your eye or anything else. Wash your hands before use.
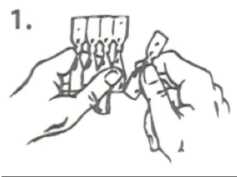

1. Tear one single-dose container from the strip.
2. Hold the single-dose container upright (with the top uppermost) and twist off the top.
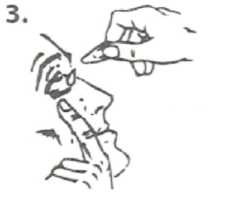
3. Gently pull down the lower eyelid to form a pocket.
Turn the single-dose container upside down and squeeze it to release one drop into each eye. Blink your eyes a few times.
Do not re-use the single-dose container even if there is some solution left. It is most important that you throw it away and do not keep it.
If you use more Carmellose sodium than you should
It will not cause you any harm. If you are worried, talk to your doctor or pharmacist.
If you forget to use Carmellose sodium
Use a single drop in each eye that needs treatment as soon as you remember, and then go back to your regular routine. Do not use a large number of drops to make up for forgetting to put drops in your eye or eyes earlier.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
^ Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The side effects are classified into the following categories, depending on how often they occur:
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
^ How to store Carmellose sodium
Keep out of the sight and reach of children.
Do not store above 25oC
Do not use Carmellose sodium after the expiry date which is stated on the single-dose container tab and the carton after EXP:. The expiry date refers to the last day of that month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
|
Very common |
may affect more than 1 in 10 people |
|
Common |
may affect up to 1 in 10 people |
|
Uncommon |
may affect up to 1 in 100 people |
|
Rare |
may affect up to 1 in 1,000 people |
|
Very rare |
may affect up to 1 in 10,000 people |
|
Not known |
frequency cannot be estimated from the available data |
The following side effects may be seen with Carmellose sodium.
|
Common |
Eye irritation (including burning and discomfort), eye pain, itchy eyes, visual disturbance. |
|
Not known |
Allergic reactions (including eye allergy), blurred vision, sticky eye, watery eye, redness of the eye, eye injury to the surface of the eye due to the tip of the vial touching the eye during use. |
f6 Contents of the pack and other information
What Carmellose sodium contains
The active ingredient is called carmellose sodium 5mg/ml.
The other ingredients are sodium chloride, sodium lactate, potassium chloride, calcium chloride, magnesium chloride and purified water.
The ingredients and the amount used in Carmellose sodium were chosen to match what appears in tears produced naturally by the eye.
What Carmellose sodium looks like and contents of the pack
Carmellose sodium is a clear colourless eye drop solution, in a single-dose container. The single-dose container has a twist off top.
Each single-dose container contains 0.4ml of solution.
Each pack contains 30 single-dose containers.
Manufacturer and Licence Holder
This medicine is manufactured by Allergan Pharmaceuticals Ireland, Westport, County Mayo, Ireland and is procured from within the EU. Product Licence Holder LTT Pharma Limited, Unit 18, Oxleasow Road, East Moons Moat, Redditch, Worcestershire, B98 0RE. Repackaged by Lexon (UK) Limited, B98 0RE, UK.
If you have any questions or are not sure about anything, ask your doctor or pharmacist. They will have additional information about this medicine and will be able to advise you.
[P PL 33723/0107
Revision date: 23/09/16
Blind or partially sighted?
Is this leaflet hard to see or read? Phone Lexon (UK) Limited, Tel: 01527 505414 for help.
Ref: 0107/230916/2/B