Celluvisc 1% W/V Eye Drops Solution
Out of date information, search anotherPACKAGE LEAFLET: INFORMATION FOR THE USER
Celluvisc® 1% w/v eye drops, solution
(carmellose sodium)
The name of your medicine is Celluvisc 1 % w/v eye drops, solution, solution but will be referred to as Celluvisc throughout this leaflet.
Read all of this leaflet carefully because it contains important information for you.
This medicine is available without prescription. However, you still need to use Celluvisc carefully to get the best results from it.
• Keep this leaflet. You may need to read it again.
• Ask your pharmacist if you need more information or advice.
• You must contact a doctor if your symptoms worsen or do not improve.
• If any of the side effects gets serious, or if you notice any side effect not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Celluvisc is and what it is used for
2. Before you use Celluvisc
3. How to use Celluvisc
4. Possible side effects
5. How to store Celluvisc
6. Further information
1. WHAT CELLUVISC IS AND WHAT IT IS USED FOR Celluvisc is a tear substitute and contains the lubricant called carmellose sodium. It is used for the treatment of the symptoms of dry eye (such as soreness, burning, irritation or dryness).
2. BEFORE YOU USE CELLUVISC Do not use Celluvisc
• If you are allergic (hypersensitive) to carmellose sodium or any of the other ingredients of Celluvisc (see section 6 for a full list of ingredients).
Take special care with Celluvisc
• If you wear contact lenses, these should be removed before using Celluvisc. The lenses can be put back again 15 minutes after you have applied your drops.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
If you are using other eye drops, leave at least 15 minutes before putting in Celluvisc.
Pregnancy and breast-feeding
Celluvisc can be used during pregnancy and breast-feeding. Driving and using machines
Celluvisc may cause short-lasting blurring of vision typically lasting 1-15 minutes. If you do experience temporary blurring, do not drive or use machines until your sight is clear.
3. HOW TO USE CELLUVISC
Celluvisc is for ocular use (applied on the eye).
Follow these instructions unless the pharmacist or your doctor gave you different advice.
The usual dose is 1 -2 drops of Celluvisc in the affected eyes as needed.
Make sure that the single-dose container is intact before use. The solution should be used immediately after opening. To avoid contamination, do not let the open-end of the single-dose container touch your eye or anything else. Wash your hands before use.
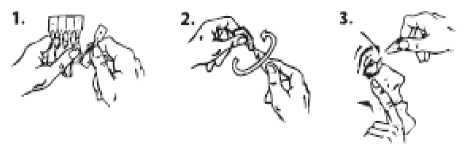
1. Tear one single-dose container from the strip.
2. Hold the single-dose container upright (with the cap uppermost) and twist off the cap.
3. Gently pull down the lower eyelid to form a pocket. Turn the single-dose container upside down and squeeze it to release one drop into each eye. Blink your eyes a few times.
Do not re-use the single-dose container even if there is some solution left.
If irritation, pain, redness or changes in vision occur or if you feel your condition is getting worse, stop taking this medicine and consult your doctor or pharmacist.
If you use more Celluvisc than you should
It will not cause you any harm. If you are worried, talk to your doctor or pharmacist.
If you forget to use Celluvisc
Apply your next dose as required or at the normal time as directed by your pharmacist or doctor. Do not take a double dose to make up for forgotten individual doses.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Celluvisc can cause side effects, although not everybody gets them.
The following side affects are known to occur, but the number of people likely to be affected is unknown:
• Eye irritation, burning or stinging sensation,
• Blurring of vision,
• Increase in tear production (also known as tearing).
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE CELLUVISC
Keep out of the sight and reach of children.
Do not store above 25°C.
Do not use Celluvisc after the expiry date which is stated on the labels after ‘Exp'. The expiry date refers to the last day of that month.
Do not use if you notice that the vial cap has already broken-off. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION What Celluvisc contains
• The active substance is: Carmellose sodium 1% w/v.
• Other ingredients: Sodium chloride, sodium lactate, potassium chloride, calcium chloride & purified water.
What Celluvisc looks like and contents of the pack Celluvisc is a clear, colourless to slightly yellow viscous solution in plastic vials. Each vial contains 0.4ml of solution and has a twist-off ‘cap'.
Celluvisc comes in pack sizes of 30 vials.
Manufactured by: Allergan Pharmaceuticals Ireland, Westport, Co. Mayo, Ireland.
Procured from within the EU and repackaged by the Product Licence holder: B&S Healthcare, Unit 4, Bradfield Road, Ruislip, Middlesex, HA4 0NU.
Celluvisc® 1% w/v eye drops, solution PL No: 18799/1404
Leaflet date: 23.12.2014 P
Celluvisc is a registered trademark of Allergan Inc.