Clarelux 500Micrograms/G Cutaneous Foam In Pressurised Container
CLARELUX 500 mg/g - Notice - Format : 185 x 350 mm - Taille du texte : 9 pt, interl. 10,8 pt Version UK - Dossier 4609 - 20/02/2015

m
PACKAGE LEAFLET: INFORMATION FOR THE USER
500 micrograms/g
cutaneous foam in pressurised container


Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or your pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What CLARELUX® is and what it is used for
2. What you need to know before you use CLARELUX®
3. How to use CLARELUX®
4. Possible side effects
5. How to store CLARELUX®
6. Contents of the pack and other information
1. What CLARELUX® is and what it is used for
CLARELUX® contains the active substance clobetasol propionate which belongs to a group of medicines known as topical corticosteroids. CLARELUX® is a highly potent topical corticosteroids.
CLARELUX® is a foam to be applied to the skin. CLARELUX® is used as a short-term treatment for scalp conditions, e.g. psoriasis of the scalp, which do not respond satisfactorily to weaker corticosteroids.
2. What you need to know before you use CLARELUX®
Do not use CLARELUX®:
• If you are allergic to clobetasol propionate, to other corticosteroids or any of the other ingredients of CLARELUX®;
• If you have an infectious skin disease, either viral (e.g. herpes, shingles, chickenpox...), bacterial (e.g. impetigo ...), fungal (caused by microscopic fungi) or parasitic;
• If you suffer from burns, ulcerated lesions or other skin condition such as rosacea, acne, skin inflammation around the mouth, itching (pruritus) around the anus or genitals.
• On any area of your body or face (included the eyelids), apart from your scalp.
Warnings and precautions
Talk to your doctor or pharmacist before using
CLARELUX®.
Stop treatment immediately and talk to your doctor if an allergic reaction occurs, signs of which may include skin rash, itching or painless tissue swelling (oedema).
As with all topical corticosteroids, CLARELUX® can be absorbed through the skin and can cause side effects such as adrenocortical suppression - see Section 4 for all possible side effects. Due to this:
• Long-term treatment with CLARELUX® should be avoided;
• CLARELUX® should not be applied to a large surface area;
• The treated areas should not be bandaged or covered unless directed by your doctor;
• The use of CLARELUX® on wounds or ulcerations is not recommended.
Inform your doctor:
• If your condition does not improve after 2 weeks of treatment.
• If an infection occurs, as this may require discontinuation of treatment with CLARELUX®.
• If you start to experience problems with your vision,
as this type of medicine may increase the development of cataracts and glaucoma.
Wash your hands carefully after each application.
In the event of accidental contact with the face or eyes, rinse thoroughly with plenty of water.
Children and adolescents
Treatment is not recommended in children and
adolescents.
Other medicines and CLARELUX®
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. CLARELUX® should not be used during pregnancy or breast-feeding unless advised by your doctor.
Driving and using machines
CLARELUX® should not affect your ability to drive or
operate machines.
Important information about some of the ingredients in CLARELUX®
This medicine contains propylene glycol, which may cause skin irritation. It also contains cetyl and stearyl alcohol, which may cause local skin reactions (e.g. contact dermatitis).
3. How to use CLARELUX®
WARNINGS:
The canister contains a pressurised, flammable liquid.
Do not use or store near a naked flame, source of ignition, any heat generating material or electrical device in use.
Do not smoke whilst using or holding this can.
Always use CLARELUX® exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Use this medication only for the condition for which it was prescribed. CLARELUX® must only be applied to the scalp and should not be swallowed.
Dispensing directly onto hands is not recommended, as the foam will begin to melt immediately upon contact with warm skin.
Apply CLARELUX® to the affected area of the scalp twice a day, once in the morning and once at night, as
follows:
Attention: for proper dispensing of foam, it is important to hold the container upside down!
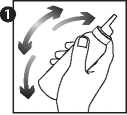
1. Shake the can well.
2. Turn the can upside down and squirt a small amount (the size of a walnut) either directly onto the scalp, or into the cap of the can, onto a saucer or other cool surface and then onto the scalp.
CLARELUX® should always be applied thinly, so use as little as possible when covering the affected areas. The exact amount you need depends on the size of the affected area.
Do not apply to the eyelids and take care to avoid contact with eyes, nose, and mouth.
Do not squirt CLARELUX® onto your hands, as the foam will begin to melt immediately upon contact with warm skin.
3. Move the hair away from the foam and gently massage into the scalp, until it disappears and is absorbed. Repeat if necessary, to treat the entire affected area.
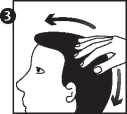
Wash your hands after applying CLARELUX® and discard any unused foam.
Do not use CLARELUX® on your face. If some foam accidentally gets into your eyes, nose or mouth, rinse immediately with cold water. You may feel a stinging sensation. Contact your doctor, if the pain continues.
The treated areas should not be bandaged or covered unless directed by your doctor.
Do not wash or rinse the treated scalp areas immediately after applying CLARELUX®.
• The canister contains a pressurised, flammable liquid.
• Do not store near a naked flame, source of ignition, any heat generating material or electrical device in use.
• Do not expose to temperatures higher than 50°C or to direct sunlight.
• Do not pierce or burn the can even when empty.
• When you have finished your treatment, dispose of the can safely.
Do not use more than 50g of CLARELUX® foam per week. Treatment should not be given for more than 2 weeks. After this period CLARELUX® may be used occasionally if needed. Alternatively your doctor may prescribe a weaker steroid to control your condition.
If you use more CLARELUX® than you should If you use CLARELUX® Foam in a larger quantity or for a long period of time without your doctor's knowledge you should tell your doctor immediately.
If you forget to use CLARELUX®
Use it as soon as you remember, then continue as before. If you only remember at the time of your next dose, use a single dose and continue as before (do not apply a double dose to make up for the forgotten dose). If you miss several doses, tell your doctor.
If you stop using CLARELUX®
Do not stop using CLARELUX® suddenly as this may harm you. Your doctor may need to discontinue the treatment gradually and you may need regular check-ups.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, CLARELUX® can cause side effects, although not everybody gets them.
Stop using CLARELUX® and contact your doctor immediately if hypersensitivity reactions occur, such as local irritation.
The side effects may include:
Common side effects (occurring in less than 1 in 10 people but more than 1 in 100):
• Burning sensation
• Other skin reaction when applied to the skin
Very rare effects (occurring in less than 1 in 10,000 people):
• Sensation of tingling or pricking
• Eye irritation
• Swollen veins
• Skin irritation and tenderness
• Skin tightness
• Itchy skin rash (contact dermatitis)
• Aggravated scaly skin rash (psoriasis aggravated)
• Redness at the application site
• Itching and sometimes with pain at the application site
• Presence of blood, protein and nitrogen in your urine may be detected by a doctor
Additional side effects may include:
• Changes in hair growth (abnormal hair growth away from the application site and on unusual parts of the body )
• Changes in skin colour
• Irritation of the hair follicules e.g. pain, heat and redness
• Mouth rashes
• Redness and eruptions on the face
• Delay in wound healing
• Effects on the eyes (cataract, high pressure in the eye)
Side effects caused by prolonged use include:
• White markings on skin (striae) and dilatation of the blood vessels of the skin
• As with other topical corticosteroids, when CLARELUX® is used in large amounts and for a long period of time, this can lead to a disorder called Cushing's syndrome which symptoms include a red, puffy and rounded face (called a moon face), high blood pressure, weight gain and changes in sugar levels in the blood and urine.
• Prolonged treatment with steroids may cause thinning of the skin.
In rare instances, treatment of psoriasis with corticosteroids (or on stopping treatment) may make the condition worse and a pustular form of the disease may occur. On stopping treatment with corticosteroids, sometimes, the scalp condition may return. Also pre existing infections may worsen if CLARELUX® is not used according to the instructions.
Reporting of side effects
If you get any side effects, talk to your doctor or
pharmacist. This includes any possible side effects
not listed in this leaflet. You can also report side effects
directly via
Ireland
HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2
Tel: +353 1 6764971, Fax: +353 1 6762517, Website: www.hpra.ie, e-mail: medsafety@hpra.ie
United Kingdom
Yellow Card Scheme ,
Website: www.mhra.gov.uk/vellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store CLARELUX®
Keep out of the sight and reach of children.
Do not use CLARELUX® after the expiry date which is stated on the can and the outer carton after EXP.
The expiry date refers to the last day of that month.
Do not store above 25°C. Do not refrigerate. Store upright.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6. Contents of the pack and other information What CLARELUX® contains
1 g of cutaneous foam CLARELUX® contains
500 micrograms of clobetasol propionate as active
substance.
The other ingredients are: ethanol anhydrous, purified water, propylene glycol, cetyl alcohol, stearyl alcohol, polysorbate 60, citric acid anhydrous, potassium citrate and a propane/n-butane/isobutane propellant mixture. What CLARELUX® looks like and contents of the pack CLARELUX® is a cutaneous white foam in pressurised container. Each can contains 50 or 100 grams.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
PIERRE FABRE DERMATOLOGIE
45 PLACE ABEL-GANCE
92100 BOULOGNE
FRANCE
Manufacturer
AEROSOL SERVICE ITALIANA S.R.L. (ASI)
VIA DEL MAGLIO, 6 23868 VALMADRERA (LC)
ITALY
This medicinal product is authorised in the Member States of the EEA under the following names: CLARELUX® 500 micrograms/g cutaneous foam in Austria, Belgium, Czech Republic, France, Germany, Greece, Ireland, Luxemburg, The Netherlands, Poland, Portugal, Slovakia, United-Kingdom and Spain.
OLUX® 500 micrograms/g cutaneous foam in Italy.
Other formats: To listen to or request a copy of this leaflet in Braille, large print or audio please call,
United Kingdom: 0800 198 5000
Ireland: +44 (0) 1733 375370
Please be ready to give the following information:
Clarelux foam
For United Kingdom: PL 20693/0004 - For Ireland: PA 1230/1/1
This is a service provided by the Royal National Institute of the Blind.
Clarelux is a trademark of Pierre Fabre Dermatologie. Sold under Stiefel License - Patent n° GB 9504265
This leaflet was last approved in 01/2015.
(§)
Pierre Fabre
000 000