Clexane 60Mg/0.6Ml Syringes
Out of date information, search anotherProduct Licence holder
Procured from within the EU and repackaged by the Product Licence holder: S&M Medical Ltd, Chemilines House, Alperton Lane, Wembley, HA01DX.
Manufacturer
This product is manufactured by either of the below manufacturers:
• Sanofi Winthrop Industrie, 180 Rue Jean Jaures, 94702 Maisons-Alfort, France.
• Sanofi Winthrop Industrie, Bd. Industrial, 76580 Le Trait, France
• Chinoin Pharmaceutical and Chemical Works Private Co. Ltd, Csanyikvolgy Site, Miskolc, Csanyikvolgy, H-3510 Hungary.
POM PL No: 19488/1630
Leaflet revision date: 1 August 2014
Clexane is a registered trade mark of Aventis Pharma SA.
Is this leaflet hard to see or read? Phone 01483 505515 for help
S1630 LEAFLET Clexane 20140801
Other side effects that you should discuss with your nurse or doctor if you are concerned about them:
Very common (affects more than 1 in 10 people)
• Changes in the results of blood tests done to check how your liver is working. These usually go back to normal after you stop having Clexane.
Rare (affects less than 1 in a 1000 people)
• Changes in the potassium levels in your blood. This is more likely to happen in people with kidney problems or diabetes. Your doctor will be able to check this by carrying out a blood test.
Frequency unknown
• If Clexane is used for a long period of time (more than 3 months), it may increase the risk of you getting a condition called ‘osteoporosis'. This is when your bones are more likely to break
• Headache
• Hair loss
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Clexane
• KEEP OUT OF THE SIGHT AND REACH OF CHILDREN.
• Do not store above 25°C. Do not refrigerate or freeze.
• Do not use Clexane after the expiry date which is stated on the carton. The expiry date refers to the last day of that month.
• If the product becomes discoloured or show any other signs of deterioration, you should consult your pharmacist who will advise you what to do.
• Medicines should not be disposed of via wastewater or household waste. If you are using this medicine at home you will be given a container (a sharps bin) to use for disposal. Return the sharps bin or any used or unused syringes to your doctor or nurse or pharmacist for disposal. These measures will help to protect the environment.
6. Further information
What Clexane contains
• Each pre-filled syringe contains 20mg of enoxaparin sodium (equivalent to 2,000 IU anti-Xa activity) in 0.2ml water for injection.
• Each pre-filled syringe contains 40mg of enoxaparin sodium (equivalent to 4,000 IU anti-Xa activity) in 0.4ml water for injection
• Each pre-filled syringe contains 60mg of enoxaparin sodium (equivalent to 6,000 IU anti-Xa activity) in 0.6ml water for injection
• Each pre-filled syringe contains 80mg of enoxaparin sodium (equivalent to 8,000 IU anti-Xa activity) in 0.8ml water for injection
• Each pre-filled syringe contains 100mg of enoxaparin sodium (equivalent to 10,000 IU anti-Xa activity) in 1 ml water for injection
• Clexane also contains water for injection.
What Clexane looks like and contents of the pack
Clexane is a clear solution in a Type I glass pre-filled syringe fitted with an
injection needle and grey cap.
• Clexane 20mg/0.2ml Syringes have a white plunger
• Clexane 40mg/0.4ml Syringes have a yellow plunger
• Clexane 60mg/0.6ml Syringes have an orange plunger
• Clexane 80mg/0.8ml Syringes have a brown plunger
• Clexane 100mg/1 ml Syringes have a black plunger
Clexane is available in packs of 10 pre-filled syringes.
S1630 LEAFLET Clexane 20140801
PACKAGE LEAFLET: INFORMATION FOR USER CLEXANE® 20mg/0.2ml SYRINGES CLEXANE® 40mg/0.4ml SYRINGES CLEXANE® 60mg/0.6ml SYRINGES CLEXANE® 80mg/0.8ml SYRINGES CLEXANE® 100mg/1ml SYRINGES (enoxaparin sodium)
Your medicine is known with either of the above names but will be referred to as Clexane throughout the following leaflet.
Information for other strength of Clexane is also available in this leaflet.
Read all of this leaflet carefully before you start using this medicine
• Keep this leaflet. You may need to read it again
• If you have any further questions, ask your doctor or pharmacist
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours
• If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist
In this leaflet:
1. What Clexane is and what it is used for
2. Before you use Clexane
3. How to use Clexane
4. Possible side-effects
5. How to store Clexane
6. Further information
1. What Clexane is and what it is used for
The name of your medicine is Clexane 20mg, 40mg, 60mg, 80mg and 100mg Pre-filled Syringes (called Clexane in this leaflet). Clexane contains a medicine called enoxaparin sodium. This belongs to a group of medicines called Low Molecular Weight Heparins.
Clexane works in two ways.
1) Stopping existing blood clots from getting any bigger. This helps your body to break them down and stop them causing you harm.
2) Stopping blood clots forming in your blood.
Clexane can be used to:
• Treat blood clots that are in your blood
• Stop blood clots forming in your blood in the following situations:
• Unstable angina (where not enough blood gets to your heart)
• After an operation or long periods of bed rest due to illness
• After you have had a heart attack
• Stop blood clots forming in the tubes of your dialysis machine (used for people with kidney problems)
2. Before you use Clexane
Do not have this medicine and tell your doctor, pharmacist or nurse if:
x You are allergic (hypersensitive) to enoxaparin sodium or any of the other ingredients of Clexane (listed in Section 6: Further information)
Signs of an allergic reaction include: a rash, swallowing or breathing problems, swelling of your lips, face, throat or tongue
x You are allergic to heparin or other Low Molecular Weight Heparins such as tinzaparin or dalteparin
x You have a problem with bruising or bleeding too easily x You have an ulcer in your stomach or gut (intestine) x You have had a stroke caused by bleeding in the brain x You have an infection in your heart
x You are using the medicine called heparin to treat blood clots Do not have this medicine if any of the above apply to you. If you are not sure, talk to your doctor, pharmacist or nurse before having Clexane.
Take special care with Clexane
Check with your doctor or pharmacist or nurse before using this medicine if:
▲ You have high blood pressure
▲ You have kidney problems
▲ You have had a heart valve fitted
▲ You have ever had bruising and bleeding caused by the medicine ‘heparin'
▲ You have ever had a stroke
▲ You have ever had a stomach ulcer
▲ You have recently had an operation on your eyes or brain
▲ You are a diabetic or have a illness known as ‘diabetic retinopathy' (problems with the blood vessels in the eye caused by diabetes)
▲ You have any problems with your blood
▲ You are underweight or overweight
▲ You are elderly (over 65 years old) and especially if you are aged over 75 years old
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist or nurse before using Clexane.
Taking or using other medicines
Please tell your doctor, pharmacist or nurse if you are taking or have recently taken any other medicines. This includes medicines you buy without a prescription, including herbal medicines. This is because Clexane can affect the way some other medicines work. Also some medicines can affect the way Clexane works.
In particular, do not have this medicine and tell your doctor if:
x You are using the medicine called heparin to treat blood clots
Tell your doctor if you are taking any of the following medicines:
• Warfarin - used for thinning the blood
• Aspirin, dipyridamole, clopidogrel or other medicines - used to stop blood clots forming
• Dextran injection - used as a blood replacer
• Ibuprofen, diclofenac, ketorolac or other medicines - used to treat pain and swelling in arthritis and other illnesses
• Prednisolone, dexamethasone or other medicines - used to treat asthma, rheumatoid arthritis and other conditions
• Water tablets (diuretics) such as spironolactone, triamterene or amiloride. These may increase the levels of potassium in your blood when taken with Clexane
Your doctor may change one of your medicines or take regular blood tests to check that taking these medicines with Clexane is not causing you any harm.
Operations and anaesthetics
If you are going to have a spinal puncture or an operation where an epidural or spinal anaesthetic is used, tell your doctor that you are using Clexane. Tell also your doctor if you have any problem with your spine or if you have ever had spinal surgery.
Pregnancy and breast-feeding
Talk to your doctor before you use this medicine if you are pregnant, might become pregnant, or think you may be pregnant.
You should not use this medicine if you are pregnant and have a mechanical heart valve as you may be at increased risk of developing blood clots. Your doctor should discuss this with you.
You should not breast-feed whilst using Clexane. If you are planning to breast-feed, talk to your doctor, pharmacist or nurse.
Ask your doctor or pharmacist for advice before taking any medicine if you are pregnant or breast-feeding.
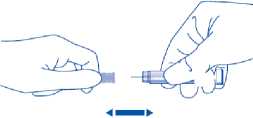
4) Carefully pull off the needle cap from the Clexane syringe. Throw away the cap. The syringe is pre-filled and ready to use.
3. How to use Clexane
Having this medicine
• Before you use Clexane your doctor or nurse may carry out a blood test
• While you are in hospital your doctor or nurse will normally give you Clexane. This is because it needs to be given as an injection
• When you go home you may need to continue to use Clexane and give it to yourself (see below instructions on how to do this)
• Clexane is usually given by injection underneath the skin (subcutaneous)
If you are not sure why you are receiving Clexane or have any questions
about how much Clexane is being given to you, speak to your doctor,
pharmacist or nurse.
How much will be given to you
• Your doctor will decide how much to give you. The amount of Clexane given to you will depend on the reason it is being used
• If you have problems with your kidneys, you may be given a smaller amount of Clexane
1) Treating blood clots that are in your blood
• The usual dose is 1.5mg for every kilogram of your weight, each day
• Clexane will usually be given for at least 5 days
2) Stopping blood clots forming in your blood in the following situations:
a) Unstable angina
• The usual amount is 1 mg for every kilogram of weight, every 12 hours
• Clexane will usually be given for 2 to 8 days. Your doctor will normally ask you to take aspirin as well
b) After an operation or long periods of bedrest due to illness
The usual dose is 20mg or 40mg each day. The dose will depend on how likely you are to develop a clot
• If you have a low to medium risk of getting a clot, you will be given 20mg of Clexane each day. If you are going to have an operation, your first injection will usually be given 2 hours before your operation
• If you have a higher risk of getting a clot, you will be given 40mg each day. If you are going to have an operation, your first injection will usually be given 12 hours before your operation
• If you are bedridden due to illness, you will be normally be given 40mg of Clexane each day for 6 to 14 days
c) After you have had a heart attack Clexane can be used for two different types of heart attack called NSTEMI or STEMI.
The amount of Clexane given to you will depend on your age and the kind of heart attack you have had.
i) NSTEMI type of heart attack
• The usual amount is 1 mg for every kilogram of weight, every 12 hours
• Clexane will usually be given for 2 to 8 days. Your doctor will normally ask you to take aspirin as well
ii) STEMI type of heart attack
If you are under 75 years old
• 30mg of Clexane will be given as an injection into your vein (intravenous injection using Clexane Multidose Vial or 60, 80 or 100mg Pre-filled syringes)
• At the same time, you will also be given Clexane as an injection under your skin (subcutaneous injection). The usual dose is 1 mg for every kilogram of your weight.
• Then you will be given 1 mg for every kilogram of your weight every 12 hours after that
• The maximum amount of Clexane given for the first two injections is 100mg
• The injections will normally be given for up to 8 days
If you are aged 75 years or older
• Your doctor or nurse will give you injections of Clexane under your skin (subcutaneous injection)
• The usual dose is 0.75mg for every kilogram of your weight, every 12 hours
• The maximum amount of Clexane given for the first two injections is 75mg
For patients having an operation called Percutaneous Coronary Intervention (PCI)
• Depending on when you were last given Clexane, your doctor may decide to give an additional dose of Clexane before a PCI operation. This is by injection into your vein (intravenous using Clexane Multidose Vial or 60, 80 or 100mg Pre-filled syringes)
3) Stop blood clots forming in the tubes of your dialysis machine
• The usual dose is 1 mg for every kilogram of your weight
• Clexane is added to the tube leaving the body (arterial line) at the start of the dialysis session
• This amount is usually enough for a 4 hour session. However, your doctor may give you a further dose of 0.5 to 1 mg for every kilogram of your weight if necessary
How to give yourself an injection of Clexane
If you are able to give Clexane to yourself, your doctor or nurse will show you how to do this. Do not try to inject yourself if you have not been trained how to do so. If you are not sure what to do, talk to your doctor or nurse immediately.
Before injecting yourself with Clexane
■ Check the expiry date on the medicine. Do not use if the date has passed
■ Check the syringe is not damaged and the medicine in it is a clear solution. If not, use another syringe
■ Make sure you know how much you are going to inject
■ Check your abdomen to see if the last injection caused any redness, change in skin colour, swelling, oozing or is still painful, if so talk to your doctor or nurse
■ Decide where you are going to inject the medicine. Change the place where you inject each time from the right to the left side of your stomach. Clexane should be injected just under the skin on your stomach, but not too near the belly button or any scar tissue (at least 5 cm away from these)
Instructions on injecting yourself with Clexane:
1) Wash your hands and the area that you will inject with soap and water. Dry them.
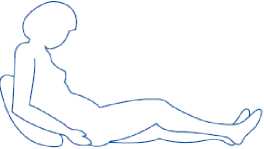
2) Sit or lie in a comfortable position so you are relaxed. Make sure you can see the place you are going to inject. A lounge chair, recliner, or bed propped up with pillows is ideal.
3) Choose an area on the right or left side of your stomach. This should be at least 5 centimetres away from your belly button and out towards your sides.
Remember: Do not inject yourself within 5 centimetres of your belly button or around existing scars or bruises. Change the place where you inject between the left and right sides of your stomach, depending on the area you were last injected.
Do not press on the plunger before injecting yourself to get rid of air bubbles. This can lead to a loss of the medicine. Once you have removed the cap, do not allow the needle to touch anything. This is to make sure the needle stays clean (sterile).
5) Hold the syringe in the hand you write with (like a pencil) and with your other hand, gently pinch the cleaned area of your abdomen between your forefinger and thumb to make a fold in the skin

Make sure you hold the skin fold throughout the injection.
6) Hold the syringe so that the needle is pointing downwards (vertically at a 90° angle). Insert the full length of the needle into the skin fold
7) Press down on the plunger with your finger. This will send the medication into the fatty tissue of the stomach. Make sure you hold
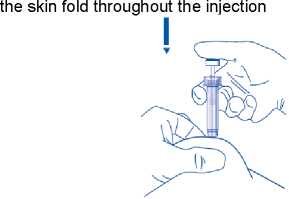
8) Remove the needle by pulling it straight out. Carefully replace the cap. You can now let go of the skin fold
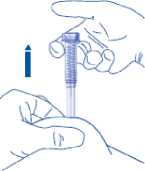
To avoid bruising, do not rub the injection site after you have injected yourself.
9) Drop the used syringe with its protective sleeve into the sharps bin provided. Close the container lid tightly and place the container out of reach of children.
When the container is full, give it to your doctor or home care nurse for disposal. Do not put it in the household rubbish.
If you have more Clexane than you should
If you think that you have used too much or too little Clexane, tell your doctor, nurse or pharmacist immediately, even if you have no signs of a problem. If a child accidentally injects or swallows Clexane, take them to a hospital casualty department straight away.
If you forget to use Clexane
If you forget to give yourself a dose, have it as soon as you remember. Do not give yourself a double dose on the same day to make up for a forgotten dose. Keeping a diary will help to make sure you do not miss a dose
If you stop using Clexane
It is important for you to keep having Clexane injections until your doctor decides to stop them. If you stop, you could get a blood clot which can be very dangerous.
Blood Tests
Using Clexane may affect the results of some blood tests. If you are going to have a blood test, it is important to tell your doctor you are having Clexane.
4. Possible side-effects
Like all medicines, Clexane can cause side effects, although not everybody gets them.
Tell a nurse or doctor or go to hospital straight away if you notice any of the following side-effects:
Very common (affects more than 1 in 10 people)
• Bleeding a lot from a wound.
Common (affects 1 to 10 people in a 100)
• A painful rash of dark red spots under the skin which do not go away when you put pressure on them. You may also notice pink patches on your skin. These are more likely to appear in the area you have been injected with Clexane.
Uncommon (affects 1 to 10 people in a 1,000)
• Sudden severe headache. This could be a sign of bleeding in the brain.
• A feeling of tenderness and swelling in your stomach. You may have bleeding inside your stomach.
Rare (affects less than 1 in a 1000 people)
• If you have an allergic reaction. The signs may include: a rash, swallowing or breathing problems, swelling of your lips, face, throat or tongue.
Frequency unknown
• If you have had a spinal puncture or a spinal anaesthetic and notice tingling, numbness and muscular weakness, particularly in the lower part of your body. Also if you lose control over your bladder or bowel (so you cannot control when you go to the toilet).
Tell a nurse or doctor as soon as possible if you notice any of the following side effects:
Common (affects 1 to 10 people in a 100)
• You bruise more easily than usual. This could be because of a blood problem (thrombocytopenia).
• You have pain, swelling or irritation in the area you have been injected with Clexane. This normally gets better after a few days.
Rare (affects less than 1 in a 1000 people)
• If you have a mechanical heart valve, treatment with Clexane might not be sufficient to prevent blood clots. You may notice that you have difficulty breathing, tiredness or difficulty exercising, chest pain, numbness, feeling sick or loss of consciousness. This could be due to a blood clot on the heart valve
Frequency unknown
• Feeling tired, faint, dizzy, having pale skin. These could be symptoms of anaemia.
• You notice yellowing of your skin or eyes and your urine becomes darker in colour. This could be a liver problem.
S1630 LEAFLET Clexane 20140801
Volume to be injected through intravenous line after dilution is completed
|
Weight |
Required dose (0.3mg/kg) |
Volume to inject when diluted to a final concentration of 3mg/ml |
|
(Kg)_ |
(mg)_ |
(ml) |
|
45 |
13.5 |
4.5 |
|
50 |
15 |
5 |
|
55 |
16.5 |
5.5 |
|
60 |
18 |
6 |
|
65 |
19.5 |
6.5 |
|
70 |
21 |
7 |
|
75 |
22.5 |
7.5 |
|
80 |
24 |
8 |
|
85 |
25.5 |
8.5 |
|
90 |
27 |
9 |
|
95 |
28.5 |
9.5 |
|
100 |
30 |
10 |
|
105 |
31.5 |
10.5 |
|
110 |
33 |
11 |
|
115 |
34.5 |
11.5 |
|
120 |
36 |
12 |
|
125 |
37.5 |
12.5 |
|
130 |
39 |
13 |
|
135 |
40.5 |
13.5 |
|
140 |
42 |
14 |
|
145 |
43.5 |
14.5 |
|
150 |
45 |
15 |
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Water for Injections
6.2 Incompatibilities Subcutaneous Injection
Clexane should not be mixed with any other injections or infusions.
Intravenous (Bolus) Injection for acute STEMI indication only
Enoxaparin sodium may be safely administered with normal saline solution (0.9%) or 5% in dextrose in water.
6.3 Shelf life
36 months
6.4 Special precautions for storage
Do not store above 25°C. Do not refrigerate or freeze.
Clexane pre-filled syringes are single dose containers - discard any unused product
6.5 Nature and contents of container
Solution for injection in Type I glass pre-filled syringes fitted with injection needle and an automatic safety device in packs of 2, 10 and 20.
6.6 Special precautions for disposal
See section 4.2 Posology and method of administration.
S1630 LEAFLET Clexane 20130814 (MIL)
THE FOLLOWING INFORMATION IS INTENDED FOR HEALTHCARE PROFESSIONALS ONLY
CLEXANE® 20mg/0.2ml SYRINGES CLEXANE® 40mg/0.4ml SYRINGES CLEXANE® 60mg/0.6ml SYRINGES CLEXANE® 80mg/0.8ml SYRINGES CLEXANE® 100mg/1ml SYRINGES (enoxaparin sodium)
Your medicine is known with either of the above names but will be referred to as Clexane throughout the following leaflet.
The following information is extracted from the SPC
Technical information for the administration of Clexane
Syringes
1 NAME OF THE MEDICINAL PRODUCT
Clexane 20mg/0.2ml Syringes Clexane 40mg/0.4ml Syringes Clexane 60mg/0.6ml Syringes Clexane 80mg/0.8ml Syringes Clexane 100mg/1 ml Syringes
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Pre-filled syringes:
20 mg Injection Enoxaparin sodium 20 mg (equivalent to 2,000 IU anti-Xa activity) in 0.2 mL Water for Injections
40 mg Injection Enoxaparin sodium 40 mg (equivalent to 4,000 IU anti-Xa activity) in 0.4 mL Water for Injections
60 mg Injection Enoxaparin sodium 60 mg (equivalent to 6,000 IU anti-Xa activity) in 0.6 mL Water for Injections
80 mg Injection Enoxaparin sodium 80 mg (equivalent to 8,000 IU anti-Xa activity) in 0.8 mL Water for Injections
100 mg Injection Enoxaparin sodium 100mg (equivalent to 10,000 IU anti-Xa activity) in 1.0 mL Water for Injections
For full list of excipients, see section 6.1
3 PHARMACEUTICAL FORM
Solution for injection.
Clear, colourless to pale yellow solution.
4.2 Posology and method of administration
Adults:
Prophylaxis of venous thromboembolism:
In patients with a low to moderate risk of venous thromboembolism the recommended dosage is 20 mg (2,000 IU) once daily by subcutaneous injection for 7 to 10 days, or until the risk of thromboembolism has diminished. In patients undergoing surgery, the initial dose should be given approximately 2 hours preoperatively. In patients with a higher risk, such as in orthopaedic surgery, the dosage should be 40 mg (4,000 IU) daily by subcutaneous injection with the initial dose administered approximately 12 hours before surgery.
Prophylaxis of venous thromboembolism in medical patients:
The recommended dose of enoxaparin sodium is 40 mg (4,000 IU) once daily by subcutaneous injection. Treatment with enoxaparin sodium is prescribed for a minimum of 6 days and continued until the return to full ambulation, for a maximum of 14 days.
Treatment of venous thromboembolism:
Clexane should be administered subcutaneously as a single daily injection of 1.5 mg/kg (150 IU/kg). Clexane treatment is usually prescribed for at least 5 days and until adequate oral anticoagulation is established.
Dosage chart for 1.5mg/kg SC treatment of DVT, PE or both
|
Patient weight |
Kg |
Syringe label |
Dose (mg) |
Injection volume (ml) |
|
100mg/ml |
40 |
60mg/0.6ml |
60 od |
0.60 |
|
Solution for |
45 |
80mg/0.8ml |
67.5 od |
0.675 |
|
Injection |
50 |
80mg/0.8ml |
75 od |
0.75 |
|
Clexane |
55 |
100mg/1 ml |
82.5 od |
0.825 |
|
syringes |
60 |
100mg/1 ml |
90 od |
0.90 |
|
65 |
100mg/1 ml |
97.5 od |
0.975 |
|
Dosage chart for 1.5mg/kg SC treatment of DVT, PE or both | ||||
|
Patient |
Kg |
Syringe label |
Dose |
Injection |
|
weight |
(mg) |
volume (ml) | ||
|
150mg/ml |
70 |
120mg/0.8ml |
105 od |
0.70 |
|
Solution for |
75 |
120mg/0.8ml |
112.5 od |
0.76 |
|
Injection |
80 |
120mg/0.8ml |
120 od |
0.80 |
|
Clexane |
85 |
150mg/1ml |
127.5 od |
0.86 |
|
Forte |
90 |
150mg/1ml |
135 od |
0.90 |
|
syringes |
95 |
150mg/1ml |
142.5 od |
0.96 |
|
100 |
150mg/1ml |
150 od |
1.00 | |
Please be aware that in some cases it is not possible to achieve an exact dose due to the graduations on the syringe and so some of the volumes recommended in this table have been rounded up to the nearest graduation.
Treatment of unstable angina and non-Q-wave myocardial infarction
The recommended dose is 1 mg/kg Clexane every 12 hours by subcutaneous injection, administered concurrently with oral aspirin (100 to 325mg once daily).
Treatment with Clexane in these patients should be prescribed for a minimum of 2 days and continued until clinical stabilisation. The usual duration of treatment is 2 to 8 days.
Dosage chart for 1mg/kg SC treatment of UA or NSTEMI
|
Patient weight |
Kg |
Syringe label |
Dose (mg) |
Injection volume (ml) |
|
40 |
40mg/0.4ml |
40 bd |
0.40 | |
|
45 |
60mg/0.6ml |
45 bd |
0.45 | |
|
50 |
60mg/0.6ml |
50 bd |
0.50 | |
|
55 |
60mg/0.6ml |
55 bd |
0.55 | |
|
100mg/ml |
60 |
60mg/0.6ml |
60 bd |
0.60 |
|
Solution |
65 |
80mg/0.8ml |
65 bd |
0.65 |
|
for |
70 |
80mg/0.8ml |
70 bd |
0.70 |
|
Injection |
75 |
80mg/0.8ml |
75 bd |
0.75 |
|
Clexane |
80 |
80mg/0.8ml |
80 bd |
0.80 |
|
syringes |
85 |
100mg/1 ml |
85 bd |
0.85 |
|
90 |
100mg/1 ml |
90 bd |
0.90 | |
|
95 |
100mg/1 ml |
95 bd |
0.95 | |
|
100 |
100mg/1 ml |
100 bd |
1.00 |
Product Licence holder
Procured from within the EU and repackaged by the Product Licence holder: S&M Medical Ltd, Chemilines House, Alperton Lane, Wembley, HA0 1DX.
I POM I PL No: 19488/1630
Leaflet revision date: 1 August 2014
S1630 LEAFLET Clexane 20140801 (MIL)
|
Dosage chart for 1mg/kg SC treatment of UA or NSTEMI | ||||
|
Patient |
Kg |
Syringe label |
Dose |
Injection |
|
weight |
(mg) |
volume (ml) | ||
|
150mg/ml |
105 |
120mg/0.8ml |
105 bd |
0.70 |
|
Solution for |
110 |
120mg/0.8ml |
110 bd |
0.74 |
|
Injection |
115 |
120mg/0.8ml |
115 bd |
0.78 |
|
Clexane |
120 |
120mg/0.8ml |
120 bd |
0.80 |
|
Forte |
125 |
150mg/1 ml |
125 bd |
0.84 |
|
130 |
150mg/1 ml |
130 bd |
0.88 | |
|
syringes | ||||
|
135 |
150mg/1 ml |
135 bd |
0.90 | |
|
140 |
150mg/1 ml |
140 bd |
0.94 | |
|
145 |
150mg/1 ml |
145 bd |
0.98 | |
|
150 |
150mg/1 ml |
150 bd |
1.00 | |
Dosage chart for 1 mg/kg SC treatment of STEMI
|
Patient weight |
Kg |
Syringe label |
Dose (mg) |
Injection volume (ml) |
|
40 |
40mg/0.4ml |
40 bd |
0.40 | |
|
45 |
60mg/0.6ml |
45 bd |
0.45 | |
|
50 |
60mg/0.6ml |
50 bd |
0.50 | |
|
55 |
60mg/0.6ml |
55 bd |
0.55 | |
|
100mg/ml |
60 |
60mg/0.6ml |
60 bd |
0.60 |
|
Solution |
65 |
80mg/0.8ml |
65 bd |
0.65 |
|
for |
70 |
80mg/0.8ml |
70 bd |
0.70 |
|
Injection |
75 |
80mg/0.8ml |
75 bd |
0.75 |
|
Clexane |
80 |
80mg/0.8ml |
80 bd |
0.80 |
|
syringes |
85 |
100mg/1 ml |
85 bd |
0.85 |
|
90 |
100mg/1 ml |
90 bd |
0.90 | |
|
95 |
100mg/1 ml |
95 bd |
0.95 | |
|
100 |
100mg/1 ml |
100 bd |
1.00 |
Please be aware that in some cases it is not possible to achieve an exact dose due to the graduations on the syringe and so some of the volumes recommended in this table have been rounded up to the nearest graduation.
Treatment of acute ST-seqment Elevation Myocardial Infarction
The recommended dose of enoxaparin sodium is a single IV bolus of 30mg plus a 1 mg/kg SC dose followed by 1 mg/kg administered SC every 12 hours (max 10Omg for the first two doses only, followed by 1 mg/kg dosing for the remaining doses). For dosage in patients >75 years of age, see section 4.2 Posology and method of administration: Elderly.
When administered in conjunction with a thrombolytic (fibrin specific or non-fibrin specific) enoxaparin sodium should be given between 15 minutes before and 30 minutes after the start of fibrinolytic therapy. All patients should receive acetylsalicylie acid (ASA) as soon as they are identified as having STEMI and maintained under (75 to 325mg once daily) unless contraindicated.
The recommended duration of enoxaparin sodium treatment is 8 days or until hospital discharge, whichever comes first.
For patients managed with Percutaneous Coronary Intervention (PCI): If the last enoxaparin sodium SC administration was given less than 8 hours before balloon inflation, no additional dosing is needed. If the last SC administration was given more than 8 hours before balloon inflation, an IV bolus of0.3mg/kg of enoxaparin sodium should be administered.
Prevention of extracorporeal thrombus formation during haemodialysis:
A dose equivalent to 1 mg/kg (100 lU/kg) introduced into the arterial line at the beginning of a dialysis session is usually sufficient for a 4 hour session. If fibrin rings are found, such as after a longer than normal session, a further dose of 0.5 to 1 mg/kg (50 to 100 lU/kg) may be given. For patients at a high risk of haemorrhage the dose should be reduced to 0.5mg/kg (50 lU/kg) for double vascular access or 0.75mg/kg (75 lU/kg) for single vascular access.
Elderly:
For treatment of acute ST-segment Elevation Myocardial Infarction in elderly patients >75 years of age, do not use an initial IV bolus. Initiate dosing with 0.75mg/kg SC every 12 hours (maximum 75mg for the first two doses only, followed by 0.75mg/kg dosing for the remaining doses).
For other indications, no dosage adjustments are necessary in the elderly, unless kidney function is impaired (see also section 4.2 Posology and method of administration: Renal impairment, section 4.4 Special warnings and precautions for use: Haemorrhage in the elderly; Renal impairment, and Monitoring', section 5.2 Pharmacokinetic properties).
|
Dosage chart for 1 mg/kg SC treatment of STEMI | ||||
|
Patient weight |
Kg |
Syringe label |
Dose (mg) |
Injection volume (ml) |
|
105 |
120mg/0.8ml (1) |
105 bd (1) |
0.70 (1) | |
|
110 |
120mg/0.8ml (1) |
110 bd (1) |
0.74 (1) | |
|
150mg/ml |
115 |
120mg/0.8ml (1) |
115 bd (1) |
0.78 (1) |
|
Solution for Injection Clexane |
120 |
120mg/0.8ml (1) |
120 bd (1) |
0.80 (1) |
|
125 |
150mg/1ml (1) |
125 bd (1) |
0.84 (1) | |
|
130 |
150mg/1ml (1) |
130 bd (1) |
0.88 (1) | |
|
135 |
150mg/1ml (1) |
135 bd (1) |
0.90 (1) | |
|
syringes |
140 |
150mg/1ml (1) |
140 bd (1) |
0.94 (1) |
|
145 |
150mg/1ml (1) |
145 bd (1) |
0.98 (1) | |
|
150 |
150mg/1ml (1) |
150 bd (1) |
1.00 (1) | |
(1) Not to be given for the first two doses - (maximum 10Omg for the first two doses only, followed by 1 mg/kg dosing for the remaining doses)
Please be aware that in some cases it is not possible to achieve an exact dose due to the graduations on the syringe and so some of the volumes recommended in this table have been rounded up to the nearest graduation.
Dosage chart for 0.75mg/kg SC treatment of STEMI (elderly patients aged >75 years only)
|
Patient weight |
Kg |
Syringe label |
0.75mg/kg Dose (mg) |
Adjusted dosing (mg) |
Injection volume (ml) |
|
40 |
60mg/0.6ml |
30 bd |
30 bd |
0.30 | |
|
45 |
60mg/0.6ml |
33.75 bd |
35 bd |
0.35 | |
|
50 |
60mg/0.6ml |
37.5 bd |
37.5 bd |
0.375 | |
|
100mg/ml |
55 |
60mg/0.6ml |
41.25 bd |
42.5 bd |
0.425 |
|
Solution |
60 |
60mg/0.6ml |
45 bd |
45 bd |
0.45 |
|
for |
65 |
60mg/0.6ml |
48.75 bd |
50 bd |
0.5 |
|
Injection |
70 |
60mg/0.6ml |
52.5 bd |
52.5 bd |
0.525 |
|
Clexane |
75 |
60mg/0.6ml |
56.25 bd |
57.5 bd |
0.575 |
|
syringes |
80 |
60mg/0.6ml |
60 bd |
60 bd |
0.60 |
|
85 |
80mg/0.8ml |
63.75 bd |
65 bd |
0.65 | |
|
90 |
80mg/0.8ml |
67.5 bd |
67.5 bd |
0.675 | |
|
95 |
80mg/0.8ml |
71.25 bd |
72.5 bd |
0.725 | |
|
100 |
80mg/0.8ml |
75 bd |
75 bd |
0.75 | |
|
105 |
80mg/0.8ml |
78.75 bd (1) |
80 bd (1) |
0.80 (1) | |
|
110 |
100mg/1ml |
82.5 bd (1) |
82.5 bd (1) |
0.825 (1) | |
|
115 |
100mg/1ml |
86.25 bd (1) |
87.5 bd (1) |
0.875 (1) | |
|
120 |
100mg/1ml |
90 bd (1) |
90 bd (1) |
0.90 (1) | |
|
125 |
100mg/1ml |
93.75 bd (1) |
95 bd (1) |
0.95 (1) | |
|
130 |
100mg/1ml |
97.5 bd (1) |
97.5 bd (1) |
0.975 (1) | |
|
150mg/ml |
135 |
120mg/0.8ml |
101.25 bd (1) |
102 bd (1) |
0.68 (1) |
|
Solution |
140 |
120mg/0.8ml |
105 bd (1) |
105 bd (1) |
0.7 (1) |
|
for Injection |
145 |
120mg/0.8ml |
108.75 bd (1) |
111 bd (1) |
0.74(1) |
|
Clexane Forte syringes |
150 |
120mg/0.8ml |
112.5 bd (1) |
114 bd (1) |
0.76 (1) |
Dosage adjustments for therapeutic dosage range
|
Standard dosing |
Severe renal impairment |
|
1 mq/kq SC twice daily |
1 mq/kq SC once daily |
|
1.5 mq/kq SC once daily |
1 mq/kq SC once daily |
|
For treatment of acute STEMI in patients <75 years of aqe | |
|
30mg-single IV bolus plus a 1mg/kg SC dose followed by 1mg/kg twice daily. (Max 100mg for each of the first two SC doses) |
30mg-single IV bolus plus a 1 mg/kg SC dose followed by 1 mg/kg once daily. (Max 100mg for first SC dose only) |
|
For treatment of acute STEMI in elderly patients >75 years of age | |
|
0.75mg/kg SC twice daily without initial bolus. (Max 75mg for each of the first two SC doses) |
1 mg/kg SC once daily without initial bolus. (Max 100mg for first SC dose only) |
Dosage adjustments for prophylactic dosage ranges
|
Standard dosing |
Severe renal impairment |
|
40 mq SC once daily |
20 mq SC once daily |
|
20 mg SC once daily |
20 mg SC once daily |
(1) Not to be given for the first two doses - (maximum 75mg for the first two doses only, followed by 0.75mg/kg dosing for the remaining doses)
Please be aware that in some cases it is not possible to achieve an exact dose due to the graduations on the syringe and so some of the volumes recommended in this table have been rounded up to the nearest graduation.
Children: Not recommended, as dosage not established.
Renal impairment: (See also section 4.4 Special warnings and precautions for use: Renal impairment and Monitoring', section 5.2 Pharmacokinetic properties).
Severe renal impairment:
A dosage adjustment is required for patients with severe renal impairment (creatinine clearance < 30 ml/min), according to the following tables, since enoxaparin sodium exposure is significantly increased in this patient population:
The recommended dosage adjustments do not apply to the haemodialysis indication.
Moderate and mild renal impairment:
Although no dosage adjustments are recommended in patients with moderate renal impairment (creatinine clearance 30-50 ml/min) or mild renal impairment (creatinine clearance 50-80 ml/min), careful clinical monitoring is advised.
Hepatic impairment: In the absence of clinical studies, caution should be exercised.
Body weight:
No dosage adjustments are recommended in obesity or low body weight (see also section 4.4 Special warnings and precautions for use: Low body weight and Monitoring', section 5.2 Pharmacokinetic properties).
Clexane is administered by subcutaneous injection for the prevention of venous thromboembolic disease, treatment of deep vein thrombosis or for the treatment of unstable angina, non-Q-wave myocardial infarction and acute ST elevation myocardial infarction (STEM I); through the arterial line of a dialysis circuit for the prevention of thrombus formation in the extra-corporeal circulation during haemodialysis; and via intravenous (bolus) injection through an intravenous line only for the initial dose of acute STEMI indication and before PCI when needed. It must not be administered by the intramuscular route.
To avoid accidental needle stick after injection, the prefilled syringes are fitted with an automatic safety device
Subcutaneous injection technique
The prefilled disposable syringe is ready for immediate use.
Clexane should be administered when the patient is lying down by deep subcutaneous injection. The administration should be alternated between the left and right anterolateral or posterolateral abdominal wall. The whole length of the needle should be introduced vertically into a skin fold held between the thumb and index finger. The skin fold should not be released until the injection is complete.
Remove the needle by pulling it straight out. Carefully replace the cap. You can now let go of the skin fold Do not rub the injection site after administration.
Intravenous /Bolus') Injection Technique (for acute STEMI indication only):
For intravenous injection, either the Multidose Vial or 60mg, 80mg or 100mg prefilled syringes can be used. Enoxaparin sodium should be administered through an intravenous line. It should not be mixed or co-administered with other medications. To avoid the possible mixture of enoxaparin sodium with all other drugs, the intravenous access chosen should be flushed with a sufficient amount of saline or dextrose solution prior to and following the intravenous bolus administration of enoxaparin sodium to clear the port of drug. Enoxaparin sodium may be safely administered with normal saline solution (0.9%) or 5% dextrose in water.
• Initial 30mg bolus
For the initial 30mg bolus, using an enoxaparin sodium graduated prefilled syringe (60, 80 or 10Omg), expel the excessive volume to retain only 30mg (0.3ml) in the syringe. The 30mg dose can then be directly injected into an injection site in the intravenous line.
• Additional bolus for PCI when last SC administration was given more than 8 hours before balloon insertion
For patients being managed with Percutaneous Coronary Intervention (PCI), an additional IV bolus of 0.3mg/kg is to be administered if last SC administration was given more than 8 hours before balloon inflation (see section 4.2 Posology and method of administration: Treatment of acute ST-segment Elevation Myocardial Infarction).
In order to assure the accuracy of the small volume to be injected, it is recommended to dilute the drug to 3mg/ml.
To obtain a 3mg/ml solution, using a 60mg enoxaparin sodium prefilled syringe, it is recommended to use a 50ml infusion bag (i.e. using either normal saline solution (0.9%) or 5% dextrose in water) as follows:
Withdraw 30ml from the infusion bag with a syringe and discard the liquid. Inject the complete contents of the 60mg enoxaparin sodium prefilled syringe into the 20ml remaining in the bag. Gently mix the contents of the bag. Withdraw the required volume of diluted solution with a syringe for administration into the intravenous line (using an appropriate injection site or port).
After dilution is completed, the volume to be injected can be calculated using the following formula [Volume of diluted solution (ml) = Patient weight (kg) x 0.1] or using the table below. It is recommended to prepare the dilution immediately before use and to discard any remaining solution immediately after use.
S1630 LEAFLET Clexane 20140801 (MIL)